The seven most common reasons Fido might limp into your clinic (Sponsored by Purina Veterinary Diets)
An overview of the diagnosis and treatment of common injuries that cause lameness in dogsIn this article, I will provide an overview of the diagnosis and treatment of these common injuries that cause lameness in dogs:> Soft tissue injuries> Stifle disease (cranial cruciate ligament rupture and patellar luxation)> Hip disease (hip dysplasia)> Elbow disease (elbow dysplasia)> Shoulder disease (soft tissue pathology and ostechondrosis dissecans)> Fractures> Arthritis
An overview of the diagnosis and treatment of common injuries that cause lameness in dogs
In this article, I will provide an overview of the diagnosis and treatment of these common injuries that cause lameness in dogs:
> Soft tissue injuries
> Stifle disease (cranial cruciate ligament rupture and patellar luxation)
> Hip disease (hip dysplasia)
> Elbow disease (elbow dysplasia)
> Shoulder disease (soft tissue pathology and ostechondrosis dissecans)
> Fractures
> Arthritis
Soft tissue injuries
Minor soft tissue injuries are one of the most common reasons for lameness in dogs. Soft tissue injuries include injuries of the muscletendon unit, termed strains, as well as injuries to ligaments, which are termed sprains. A common classification uses three categories, with first degree being a minor stretch with an intact architecture that does not result in instability and third degree being a complete disruption of the architecture. Third-degree injuries are easily diagnosed since they result in obvious dysfunction/luxation. These injuries are frequently treated surgically. First- and second-degree injuries are more difficult to diagnose and are frequently treated conservatively with pain control, rest, cryotherapy, and compression (RICE = rest, ice, compression, elevation). Rehabilitation is useful to ensure optimal healing and proper myofibril/collagen orientation during early healing and return to full function later in the repair process. Several forms of external coaptation are available to support a sprain or strain during healing. In general, custom-molded splints/casts or orthoses (www.orthopets.com) are preferable since they are more comfortable for the patient and have less risk of sore development.
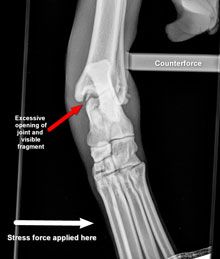
Figure 1. Stress radiographs of the tarsus showing a third-degree sprain of the collateral ligament.A strain should be suspected when evaluation of the joints and long bones does not reveal an abnormality and pain is elicited when a muscle is palpated directly or when it is stretched. To identify pathology in a specific muscle, anatomic knowledge and isolated assessment (where possible) of the muscle is needed (Table 1). “Testing” a muscle's passive flexibility (i.e. placing it under tension or stretching it) is accomplished by exerting the opposite motion of its action. Pain, heat, or swelling may be present upon palpation of the injured area. Sprains are of particular concern if they involve collateral ligaments of the major joints. Third-degree injuries of these ligaments need to be treated surgically since joint subluxation or luxation is the consequence. These injuries can usually be diagnosed by simple palpation. Stress radiographs are useful to diagnose second-degree injuries and to confirm palpatory findings. For example, a third-degree injury of the medial tarsal collateral ligament will allow for excessive opening of the medial aspect of the joint (Figure 1).
Table 1. Muscle Anatomy, Function, and Stretching
Muscle
Insertion
Function
Innervation
Stretch
Biceps brachii
Origin: Supraglenoid tubercle (scapula)
Radial and ulnar tuberosities (proximal radius + ulna)
Elbow flexion and shoulder extension
Musculo-cutaneous nerve
Elbow extension and shoulder flexion
Infraspinatus
Origin: Infraspinous fossa (scapula)
Greater tubercle (proximal humerus in between insertion of supra-spinatus and teres minor)
Shoulder flexion and extension (depending on joint angle), shoulder abduction, lateral stabilizer
Supra-scapular nerve
Shoulder extension and adduction
Triceps brachii
Origin: 4 heads
Long head: scapula
Lateral, accessory, and medial head: proximal humerus
Tuber olecranon
Elbow extension and shoulder flexion (long head only)
Radial nerve
Elbow flexion and shoulder extension (long head only)
Iliopsoas
Origin: Lumbar vertebrae
Lesser trochanter (femur)
Hip flexion and external rotation
Lumbar spinal nerves; femoral nerve
Hip extension and internal rotation
Pectineus
Origin: Iliopubic eminence and pubic tubercle via the prepubic tendon
Distomedially on the caudal femur
Limb adduction
Obturator nerve
Limb abduction
Gracilis
Origin: Pelvic symphysis
Cranial border of the tibia and tuber calcanei (part of the Achilles tendon)
Limb adduction, stifle flexion, hip extension, hock extension
Obturator nerve
Limb abduction, stifle extension, hip flexion
Semitendi-
nosus
Origin: Ischiatic tuberosity
Medial tibia and tuber calcanei (part of the Achilles tendon)
Hip extension, stifle flexion, hock extension
Sciatic nerve
Hip flexion, stifle extension
Quadriceps femoris
Origin: 4 heads
Rectus femoris: ilium
Vastii muscles: proximal femur
Tibial tuberosity
Stifle extension, hip flexion (rectus femoris only)
Femoral nerve
Stifle flexion, hip extension (rectus femoris only)
Stifle disease

Figure 2. A positive sit test. The animal on the right is unwilling to fully flex the stifle and does not sit “square,” as the dog on the left does. This may indicate cranial cruciate ligament disease.The two most common reasons for lameness associated with the stifle are cranial cruciate ligament disease (CCLD) and patellar luxation. Traditionally, the diagnosis of CCLD is based on a positive drawer and/or thrust motion test; however, animals with partial tears may not have stifle instability. Early signs of CCLD include a positive sit test (Figure 2), pain on hyperextension of the stifle (since the CCL resists hyperextension), and joint effusion. Medial buttress (thickening of the proximal, medial aspect of the tibia) is also a valuable diagnostic tool, especially for unilateral disease.
Joint effusion and degenerative changes can easily be detected on radiographs (Figure 3). Even early cases of CCLD will show some joint effusion, which makes radiographs a valuable diagnostic tool. (Be aware that small dogs may not show these radiographic changes despite having a full tear of the CCL.) A wide variety of surgical and nonsurgical treatment options are available, but the recent literature suggests that tibial plateau leveling osteotomy (TPLO) is superior to extracapsular repair and nonsurgical treatment.1,2 Surgical treatment has proven to be effective in the restoration of the limb to near normalcy, particularly after TPLO procedure.2-5 Nonsurgical treatment of CCLD is ill-defined but should include rehabilitation therapy because of the proven benefits.1,6-8 Other treatment options (such as stifle orthoses, shock wave treatment, acupuncture, stem cell therapy, and low-level laser therapy) have been recommended in non-peer-reviewed literature, but no data exist on their frequency of utilization or measures of outcome. Surgical treatment is the preferred option for active, healthy dogs regardless of degree of stifle instability (especially for young dogs). Early intervention is beneficial to decrease arthritis progression and to increase the chances of an intact meniscus at the time of surgery. TPLO in animals with early partial tears prevents further tearing of the CCL, decreases subsequent cartilage pathology, and reduces the risk of future meniscal injuries.9 For older, inactive animals, surgery is strongly encouraged if there is a meniscal tear or significant stifle instability.
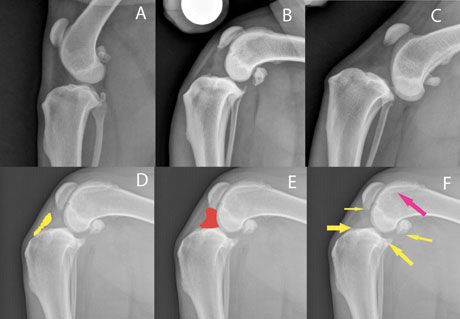
Figure 3. Radiographs of the canine stifle. A: Normal; B: Cranial cruciate ligament disease (CCLD), early partial tear without cranial tibial displacement, severe joint effusion, mild-moderate degenerative changes; C: CCLD, acute, traumatic full tear without degenerative changes but severe cranial tibial displacement; D-F: CCLD, chronic partial tear with moderate degenerative changes; the highlighted area in yellow (D) shows the fat pad; the highlighted area in red (E) shows joint effusion; and the arrows (F) indicate degenerative changes.
Patellar luxation is a commonly diagnosed orthopedic problem in small animal practice. It is frequently treated with a combination of retinacular release/imbrication, trochleoplasty, and tibial tuberosity transposition (TTT). Most patellar luxations are directed in a medial direction, even in large-breed dogs, and small-breed dogs are almost exclusively affected by medial patellar luxation.10 Lateral and traumatic luxations are uncommon. Dogs with higher-grade patellar luxation have a higher incidence of concomitant cruciate disease.11 This finding may provide additional support for early surgical treatment of (especially high-grade) patellar luxations. Femoral varus (defined as angulation of the distal femur toward the midline) plays a significant role in medial patellar luxation in large- and possibly small-breed dogs. Uncorrected femoral varus is likely a major reason for reluxation after patella surgery. Therefore, identification of patients with excessive femoral varus deformities is crucial before performing surgical correction. This should be routinely performed in large-breed dogs and is advised in higher-grade luxations in small-breed dogs.
Well-positioned craniocaudal radiographs or computed tomography (CT) scans are needed for quantitative measurement of femoral varus. The craniocaudal view should be positioned so that the fabellae are bissected by the femoral cortices, the vertical walls of the intercondylar notch are distinct parallel lines, and the lesser trochanter is only partially visible (Figure 4).
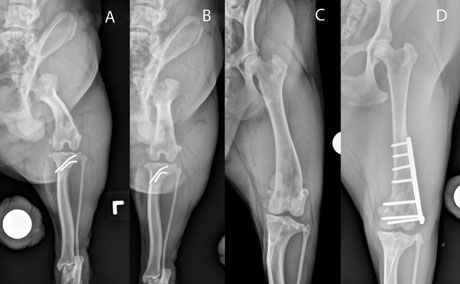
Figure 4. Radiographs of the femur to assess a dog for femoral varus. A: Incorrect positioning, demonstrating artificial femoral varus (note that the fabellae are not split by the femoral cortices and the entire lesser trochanter is visible); B: The same dog as in A with correct positioning - note that no femoral varus is present; C: A patient with femoral varus, medial patella luxation, and adequately positioned femoral alignment films; D: A patient after distal corrective osteotomy (DFO) for medial patella luxation and femoral varus.
Correction of distal femoral varus is usually accomplished by performing a distal, transverse corrective osteotomy (DFO). This technique involves removal of an adequately sized wedge (calculated based on preoperative radiographs) and internal fixation with specialized DFO plates. Since this fixation provides ideal load-sharing, the complication rate after this procedure is low.12
Hip disease
Hip dysplasia is the other common reason for hind limb lameness in dogs. It is very important to recognize diagnostic and treatment differences for patients with juvenile and adult hip dysplasia. Early diagnosis (before 5 months of age) is highly desirable since juvenile pubic symphysiodesis (JPS) is most effective in animals that are 20 weeks or younger. Therefore, screening of any large-breed puppy predisposed to hip dysplasia is indicated. Screening should include gait observation, hip extension and abduction, and an Ortolani test. If any of these findings are questionable, further diagnostics should be performed (PennHIP/OFA-like radiographs).
> Pain on hyperextension of the hip joint is observed in most animals with hip dysplasia but is not pathognomonic for hip dysplasia/arthritis. Other diseases that cause discomfort include iliopsoas injury, neurologic disease (such as LS disease), and cruciate disease. Cruciate disease can be ruled out by palpation and radiographs of the stifle. If hip abduction is not painful, hip pathology is less likely. The iliopsoas muscle is a fusion of the psoas major muscle and the iliacus muscle. It can be palpated either at the insertion at the lesser trochanter or intraabdominally (the psoas major portion originates at the L3/L4 vertebrae and can be palpated along its course ventral to the vertebral column by applying dorsal pressure through the abdominal wall). Ideally, this palpation should be performed while stretching the muscle (internal rotation, hip extension as well as spine extension).
> A positive Ortolani sign indicates hip laxity, but a positive Ortolani sign does not necessarily mean that the animal will develop coxofemoral arthritis. On the other hand, a negative Ortolani sign does not rule out hip dysplasia. False-negative tests (especially if performed without sedation) are common. As a general rule, a positive Ortolani test should be followed up with further diagnostic examination. A negative Ortolani test should not be used to rule out hip dysplasia if other signs point toward this disease.
> A routine hip-extended radiograph (OFA-like view) should be performed in any patient that is suspected to have hip dysplasia. Hip-extended radiographs that show subluxation confirm the presence of laxity. Perfectly positioned radiographs are needed to evaluate symmetry of the disease. It is important to remember that hip-extended positioning actually makes the hip joints look less subluxated because of the twisting of the joint capsule (a so-called “windup mechanism”). This means that in a puppy with significant laxity, OFA-like radiographs may actually be normal, which is why PennHIP is needed to diagnose these patients correctly (Figure 5). In a juvenile patient, PennHIP radiographs are ideal to quantify the degree of dysplasia present. (Training is required to perform this radiography: info.antechimagingservices.com/pennhip/).
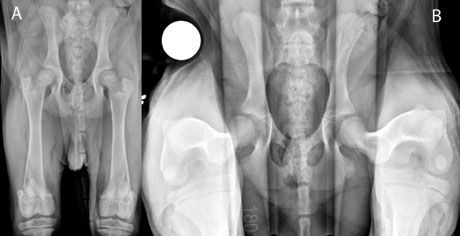
Figure 5. A: OFA-like radiographs of a 4-month-old puppy showing no clear evidence of hip dysplasia; B: PennHIP radiographs of the same patient confirming hip laxity, which triggered JPS treatment.
Animals with a PennHIP distraction index greater than 0.3 should be considered for immediate JPS surgery. JPS is a simple procedure with minimal complications that is most effective in dogs with mild to moderate laxity when performed as early as possible (16 weeks).13,14 If JPS is combined with a spay or neuter procedure, the owner should be informed about the disadvantages of early sterilization (e.g. possible decreased life span; increased risk of neoplasia, orthopedic disease).
Adult hip dysplasia is most frequently treated with medical management, femoral head and neck ostectomy (FHO), or total hip replacement. FHO should only be performed if medical management has been exhausted. The owners should be made aware that return to normal function is not always possible. Intense rehabilitation and pain management should follow FHO. Total hip replacement is an expensive procedure that is generally thought to be the gold standard of care. However, it can be associated with frustrating complications (luxation, femur fracture, and infection), which may require revision surgery or removal of the implants. Therefore, in-depth owner education is necessary before choosing a total hip replacement.
Elbow disease
Elbow dysplasia encompasses ununited anconeal process (UAP), fragmented coronoid process (FCP) or medial compartment/coronoid disease, ostechondrosis dissecans (OCD), and incongruity. Traditionally, these conditions are considered congenital and so are most common in juvenile patients. They can also occur in adult patients due to severe arthritis. Fragmentation of the coronoid process, however, also has been described in adult patients without degenerative changes.15 This presentation has been termed adult-onset FCP or traumatic FCP because of a possible traumatic etiology. Regardless of the etiology, adult dogs can suffer from coronoid disease even if radiographs appear normal.
Treatment and diagnostic steps for adult-onset and juvenile coronoid disease are similar. Treatment is not a cure, and degenerative disease will develop to some degree regardless of treatment. Therefore, lifelong medical management should be part of the treatment plan for any dog with elbow disease.
Diagnosis of elbow disease in immature animals can be challenging. Palpation of the medial compartment (while supinating or pronating the distal limb and flexing the elbow) frequently elicits pain. Radiographs should include three views: routine lateral, craniocaudal, and a hyperflexed view to isolate the anconeal process. An UAP is diagnosed if a lucent line is visible separating the anconeal process and the rest of the olecranon in dogs older than 24 weeks (Figure 6). The greater challenge, however, is to diagnose mild incongruity, OCD, and FCP. Unfortunately, radiographs have a low sensitivity for detection of FCP (~25%). A CT scan or diagnostic arthroscopy should be performed if radiographs do not provide a definitive diagnosis. Radiographs in juvenile patients with coronoid disease may show an indistinct coronoid process and ulnar sclerosis. These changes can be very subtle and may be difficult to detect (Figure 7).
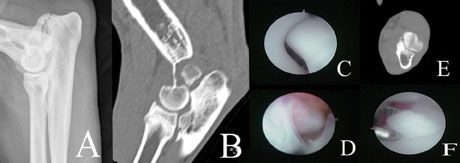
Figure 6. Images of a dog with a combination of ununited anconeal process (UAP) and coronoid disease. A: UAP as seen on radiographs; there is no clear evidence of coronoid disease; B: CT image of UAP and radioulnar incongruity; C: Normal arthroscopic appearance of anconeal process; D: Arthroscopic appearance of UAP; E: Concurrent fragmented coronoid process (FCP) diagnosed with CT; F: Arthroscopic appearance of FCP.
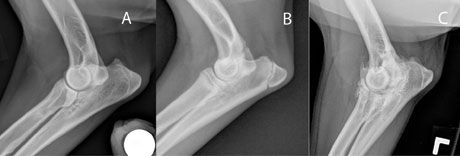
Figure 7. Radiographs of the canine elbow. A: Normal; B: Dog with coronoid disease - note the indistinct coronoid process and mild ulnar sclerosis; C: Severe elbow arthritis.
A wide variety of treatment options are available for elbow disease, and treatment depends on the degree of arthritis, incongruity, and type of disease. For UAP, excision of the ununited anconeal process, osteotomy procedures for animals with incongruity, and “lag-screw” fixation of the UAP have been reported. For coronoid disease, removal of the fragment alone or with adjacent subchondral bone (subtotal coronoidectomy) is the most common treatment. If incongruity is present, an ulnar osteotomy can be combined with this procedure.
Mature animals with severe degenerative joint disease pose a significant treatment challenge. Since many animals are bilaterally affected, amputation is not an option and elbow arthrodesis is associated with suboptimal function and a significant complication rate. If traditional medical management is exhausted, novel treatment options such as CUE (canine unicompartmental elbow arthroplasty; Arthrex) or total elbow replacement (TATE; Biomedtrix) may be considered, even though there is a lack of long-term outcome data.
Shoulder disease
Osteochondrosis dissecans (OCD) is frequently the reason for shoulder pain in immature animals. Several oblique radiographs or a CT scan may be needed for diagnosis; however, many lesions can be easily identified on routine lateral views (flattening of the caudal humeral head). OCD should be treated by removal of the cartilage flap (via arthroscopy or arthrotomy), which results in excellent function in the majority of patients.
The shoulder is the joint most commonly affected by soft tissue pathology and hence provides a diagnostic challenge. Common soft tissue injuries include biceps tendinopathy, medial shoulder instability, and myopathy of the supraspinatus muscle. Teres major, infraspinatus, and teres minor myopathies are less commonly the primary reason for lameness. Biceps tendinopathy is diagnosed by palpation (positive biceps test), ultrasonography, diagnostic arthroscopy, and magnetic resonance imaging (MRI). Radiographs or CT scans may show calcification of the muscle (Figure 8), but calcification is frequently seen with chronic injuries and may not correlate with clinical signs. The biceps brachii is a two-joint muscle that originates from the scapula and inserts on the radius/ulna. Hence, it causes shoulder extension and elbow flexion. The biceps tendon can be palpated just medial to the greater tubercle. Pain can usually be elicited when palpating and stretching the muscle at the same time. To “stretch” the biceps muscle, simultaneous shoulder flexion and elbow extension are performed. Treatment may involve intra-articular steroid (triamcinolone) injections, rehabilitation (including shock wave or laser treatment), or surgical release in severe cases that are refractory to nonsurgical management. Supraspinatus injury may be difficult to differentiate from biceps pathology, and the two diseases frequently are encountered together. Palpation of the supraspinatus is performed at the insertion at the greater tubercle; stretching of the muscle does not require elbow extension (allowing differentiation from biceps problems). The same diagnostic procedures as for biceps pathology are indicated. Treatment may include shock wave treatment, rehabilitation, and surgical release in severe cases or if impingement of the biceps tendon is present.
Medial shoulder instability is defined as injury to the medial collateral (glenohumeral) ligament and/or subscapularis tendon. This disease is recognized in people as rotator cuff injury. Severity of the disease dictates treatment; mild and moderate injuries frequently respond favorably to rehabilitation, and severe injuries require surgical reconstruction of the collateral ligaments (Table 2). Medial shoulder instability is diagnosed by palpation (increased abduction angle), diagnostic arthroscopy, and MRI. Abduction of the shoulder can be estimated during stance, but accurate abduction angle measurement requires sedation. It is important to fully extend the elbow when measuring shoulder abduction since elbow flexion will artificially increase the measurements.
Table 2. Diagnosis and Treatment of Medial Shoulder Instability
Mild
Moderate
Severe
Abduction angle
35° - 45°
45° - 65°
> 65°
Arthroscopy
Synovitis, but no obvious middle glenohumeral ligament/subscapularis pathology
Synovitis, fraying to obvious disruption of subscapularis tendon and/or middle glenohumeral ligament
Complete tearing of subscapularis and middle glenohumeral ligament
Treatment
Rehabilitation (Hobbles, Thera-band, exercises), shock wave, platelet-rich plasma
Try nonsurgical therapy first
Traumatic shoulder luxation requiring surgical reconstruction
Fractures
The goal of fracture treatment is three-fold: emergency stabilization and care of the patient, appropriate fixation of the fracture, and return to (full) function.
1) The primary concern is to address life-threatening issues (pneumothorax, hemoabdomen, etc.) and other systemic problems associated with the fracture. Pain should be assessed immediately, and treatment of pain should be given priority.
2) After successful stabilization of the patient, the goal is to accomplish appropriate fixation to allow bone healing in an adequate position. External coaptation (casting/splinting) has significant limitations when it comes to providing fracture stability. A wide variety of implants are available (Table 3). As a general rule, only external skeletal fixation, plating, and interlocking nails control all fracture forces.
3) Successful bone healing does not necessarily correlate with good clinical function. Bone healing in inadequate alignment (malunion) may cause lameness and degenerative joint disease in the long term. Soft tissue healing is also an important part of fracture healing. Early postoperative rehabilitation will reduce muscle atrophy and increase joint health and range of motion, leading to a quicker return to function.
Table 3. Implant Selection Guidelines
Fracture
Indicated Implants
Contraindicated Implants
Comments
Long-oblique, simple (two-piece) fracture of tibia, femur, humerus
Plating; Interlocking nails; External skeletal fixation; Pin and wire
External coaptation
Long-oblique fractures will result in shearing forces that can NOT be controlled sufficiently by external coaptation; Femur and humerus fractures can NOT be managed with external coaptation
Transverse, simple (two-piece) fracture of tibia, femur, humerus
Plating; External skeletal fixation; Interlocking nails
Pin and wire
Cerclage wires should only be applied to anatomically reconstructed long-oblique fractures; External coaptation may be used for tibial fractures
Comminuted fracture of tibia
Plating; External skeletal fixation; Interlocking nails
Pin and wire; External coaptation
In comminuted fractures, compressive forces can NOT be controlled with external coaptation; Pins and wire should be used only when anatomic reconstruction can be accomplished
Articular fracture (fracture line extending into the joint)
Plating; Lag-screws
External skeletal fixation; Pin and wire; External coaptation
Articular fractures need to be anatomically reduced and compressed, which can only be accomplished with screws
Transverse, simple, mid-shaft fracture of the radius (in a non-toy-breed dog)
Plating; External skeletal fixation; External coaptation
Interlocking nails; Pin and wire
It is NOT possible to apply intermedullary pins or interlocking nails to the radius without damaging the joint; hence, they should NEVER be used in the radius
Transverse, simple (two-piece) fracture of distal radius in toy-breed dogs
Plating; External skeletal fixation
Interlocking nails; Pin and wire; External coaptation
These fractures are associated with high complication rates (non-union) because of poor blood supply to the area and, hence, should not be treated with external coaptation
Arthritis
Osteoarthritis (OA) or degenerative joint disease is a common cause of lameness in animals. Diagnosis of arthritis is generally accomplished with routine radiographs.
Primary osteoarthritis is defined as osteoarthritis without an identifiable underlying pathology. Secondary osteoarthritis develops because of an underlying disease, such as stifle OA due to cruciate disease. In small animals, secondary OA is most common. The underlying reason may guide the treatment choice, such as stabilization of the stifle joint. If OA is advanced, however, the underlying disease may become the secondary problem and treatment of the original problem may not result in improvement (for example, removal of an FCP in a severely arthritic elbow).
A wide variety of OA therapies are available and should always include benign options such as weight control,16 regular activity, and joint supplements. While strong evidence for the use of nutraceuticals is available only for omega-3 fatty acid supplements,17 the absence of side effects and some evidence of beneficial effects may warrant recommendations for other nutraceuticals, such as glucosamine chondroitin sulfate. Joint diets containing high amounts of omega-3 fatty acids (e.g. Purina Veterinary Diets® JM Joint Mobility® Canine Formula) have been shown to improve subjective and objective outcome measures in dogs with naturally occurring arthritis/orthopedic disease and, hence, should be strongly suggested for these patients.18,19 Veterinary rehabilitation can be very helpful to regain muscle strength and improve joint mobility and function. There are many other options such as acupuncture, joint supplements, and novel treatment options (shock wave therapy, low-level laser treatment, stem cell and platelet-rich plasma [PRP] injections), and these can be discussed with owners. Lastly, the vast number of pain medications (e.g. NSAIDs, tramadol, gabapentin, amantadine HCl) should be considered in a multimodal treatment approach. However, pain medications should only be part of the treatment plan and not the main modality (Table 4).
Table 4. Stepwise Approach to Osteoarthritis
Factor
Example
Treatment
Step 1
Presence of underlying disease?
Cranial cruciate disease
Surgical stabilization
Step 2
Optimal weight/nutrition?
Obesity (Purina body condition score > 7)*
Weight loss diet such as Purina OM
Step 3
Adequate exercise/rehabilitation?
“Weekend warrior” (irregular, strenuous exercise); no exercise
Regular and controlled exercise, veterinary rehabilitation
Step 4
Omega-3 fatty acid supplementation?
No supplementation
Joint diet such as Purina JM or supplementation at 310 mg (EPA + DHA) per kg MBW per day
Step 5
Other nutraceuticals?
No supplementation
Supplementation with glucosamine such as Dasuquin
Step 6
Pain controlled?
Inadequate pain control
NSAIDs, tramadol, gabapentin, etc.; alternatives are acupuncture, etc.
Step 7
Polysulfated glycosaminoglycan (PSGAG)
Arthritis of one or multiple joints
Adequan injections; alternatives are pentosan polysulfate or Polyglycan
Step 8
Joint injections
One or two joints identified as major source of pain/lameness
Steroid (triamcinolone) and/or hyaluronic acid injections
Step 9
Alternative treatment options
Refractory to above treatments
Platelet-rich plasma or stem cell injections, shock wave, laser treatment, orthoses, etc.
Step 10
Surgical treatment options
Osteoarthritis of the carpal joint
Pancarpal arthrodesis
*www.purinaveterinarydiets.com or www.wsava.org/nutrition-toolkit
It is important, especially in older animals, to rule out other disease such as neoplasia. Synovial cell sarcoma may appear radiographically similar to arthritis, so it is useful to monitor arthritis progression annually. Severely arthritic joints are prone to septic arthritis. A patient with an acute worsening of a chronic lameness because of degenerative joint disease should be carefully evaluated for a secondary infectious arthritis. Clinical signs include fever, edema, periarticular swelling, and severe pain. Cytology and culture of the joint fluid should be performed, and treatment ideally should involve arthroscopic joint lavage. However, the prognosis for return to pre-injury function is poor.
Summary
Many of the commonly seen orthopedic problems are related to or cause arthritis. Any animal that undergoes joint surgery (e.g. TPLO, articular fracture repair, treatment of elbow disease) or is diagnosed with orthopedic disease that causes secondary arthritis (e.g. hip dysplasia, OCD) should be fed a high-quality joint diet containing appropriate amounts of omega-3 fatty acids (e.g. Purina Veterinary Diets JM Joint Mobility Canine Formula). If the animal is severely overweight (a body condition score [BCS] exceeding 7, on a scale of 1 to 9), it should be fed a weight loss diet (e.g. Purina Veterinary Diets® OM Overweight Management® or OM Select Blend Overweight Management™ canine formulas) with omega-3 fatty acid supplementation until a BCS of 7/9 is achieved. Owners should also be advised that lifelong maintenance of an appropriate BCS (5/9) and regular activity/rehabilitation are key features of arthritis management and prevention.
References
1. Wucherer KL, Conzemius MG, Evans R, et al. Short-term and long-term outcomes for overweight dogs with cranial cruciate ligament rupture treated surgically or nonsurgically. J Am Vet Med Assoc 2013;242(10):1364-1372.
2. Nelson SA, Krotscheck U, Rawlinson J, et al. Long-term functional outcome of tibial plateau leveling osteotomy versus extracapsular repair in a heterogeneous population of fogs. Vet Surg 2013;42(1):38-50.
3. De Medeiros M, Sanchez Bustinduy M, Radke H, et al. Early kinematic outcome after treatment of cranial cruciate ligament rupture by tibial plateau levelling osteotomy in the dog. Vet Comp Orthop Traumatol 2011;24(3):178-184.
4. Boddeker J, Druen S, Meyer-Lindenberg A, et al. Computer-assisted gait analysis of the dog: comparison of two surgical techniques for the ruptured cranial cruciate ligament. Vet Comp Orthop Traumatol 2012;25:11-21.
5. Gordon-Evans WJ, Griffon DJ, Bubb C, et al. Comparison of lateral fabellar suture and tibial plateau leveling osteotomy techniques for treatment of dogs with cranial cruciate ligament disease. J Am Vet Med Assoc 2013;243:675-680.
6. Monk ML, Preston CA, McGowan CM. Effects of early intensive postoperative physiotherapy on limb function after tibial plateau leveling osteotomy in dogs with deficiency of the cranial cruciate ligament. Am J Vet Res 2006;67:529-536.
7. Marsolais GS, Dvorak G, Conzemius MG. Effects of postoperative rehabilitation on limb function after cranial cruciate ligament repair in dogs. J Am Vet Med Assoc 2002;220(9):1325-1330.
8. Au KK, Gordon-Evans WJ, Dunning D, et al. Comparison of short- and long-term function and radiographic osteoarthrosis in dogs after postoperative physical rehabilitation and tibial plateau leveling osteotomy or lateral fabellar suture stabilization. Vet Surg 2010;39:173-180.
9. Hulse D, Beale B, Kerwin S. Second look arthroscopic findings after tibial plateau leveling osteotomy. Vet Surg 2010;39(3):350-354.
10. Alam MR, Lee JI, Kang HS, et al. Frequency and distribution of patellar luxation in dogs. 134 cases (2000 to 2005). Vet Comp Orthop Traumatol 2007;20(1):59-64.
11. Campbell CA, Horstman CL, Mason DR, et al. Severity of patellar luxation and frequency of concomitant cranial cruciate ligament rupture in dogs: 162 cases (2004-2007). J Am Vet Med Assoc 2010;236(8):887-891.
12. Weh JL, Kowaleski MP, Boudrieau RJ. Combination tibial plateau leveling osteotomy and transverse corrective osteotomy of the proximal tibia for the treatment of complex tibial deformities in 12 dogs. Vet Surg 2011;40(6):670-686.
13. Dueland RT, Adams WM, Patricelli AJ, et al. Canine hip dysplasia treated by juvenile pubic symphysiodesis. Part I: two year results of computed tomography and distraction index. Vet Comp Orthop Traumatol 2010;23(5):306-317.
14. Dueland RT, Patricelli AJ, Adams WM, et al. Canine hip dysplasia treated by juvenile pubic symphysiodesis. Part II: two year clinical results. Vet Comp Orthop Traumatol 2010;23(5):318-325.
15. Meyer-Lindenberg A, Langhann A, Fehr M, et al. Prevalence of fragmented medial coronoid process of the ulna in lame adult dogs. Vet Rec 2002;151:230-234.
16. Lawler DF, Larson BT, Ballam JM, et al. Diet restriction and ageing in the dog: major observations over two decades. Brit J Nutr 2008;99(4):793-805.
17. Vandeweerd JM, Coisnon C, Clegg P, et al. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J Vet Intern Med 2012;26(3):448-456.
18. Hansen RA, Harris MA, Pluhar GE, et al. Fish oil decreases matrix metalloproteinases in knee synovia of dogs with inflammatory joint disease. J Nutr Biochem 2008;19(2):101-108.
19. Moreau M, Troncy E, Del Castillo JR, et al. Effects of feeding a high omega-3 fatty acids diet in dogs with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr 2013;97:830–837. doi: 10.1111/j.1439-0396.2012.01325.x
Episode 67: Choosing trusted supplements
October 20th 2021In this episode of The Vet Blast Podcast, Dr Adam Christman chats with Dr Janice Huntingford about the latest insights into selecting the best supplements for your patients, including the importance of recommending and utilizing products that have a substantial amount of science and research behind them. (Sponsored by Vetoquinol)
Listen