Tickborne disease surveillance: An adaptive approach
In light of global ecologic changes altering emergence patterns of tickborne zoonoses, investigators mapped out a novel framework for scoping out the influx of ticks—and the diseases they bring—into new geographic areas.
(gabort / stock.adobe.com)

Zoonotic pathogens are moving targets. Most originate in wildlife1 as likely evidenced by the latest global threat, COVID-19. Shifts in associated disease patterns result from increased human contact with wildlife, globalization of human travel and trade, broad ecologic changes and vector/pathogen evolution.2,3
Lyme disease, or borreliosis, is the most common vector-borne illness in temperate areas of the northern hemisphere.4 Long established in southern and central Europe, northern Asia and the northeastern United States,5-7 it likely penetrated Canada over the past two decades in concert with the northward spread of its tick vector, Ixodes scapularis, and its causative agent, the bacterium Borrelia burgdorferi.8
To better understand the current approaches and major challenges associated with surveillance of emerging tickborne zoonoses, investigators from the University of Guelph’s Ontario Veterinary College used the incursion of Lyme disease into Canada as an archetype for study.9
Study design
A review of the available literature describing different disease surveillance programs honed in on the emergence of Lyme disease in Canada.10 Using two databases, PubMed and Web of Science, the investigators searched publications dating from 1990 through 2016. A total of 339 titles and abstracts were reviewed, of which 107 were determined to be relevant.
Survey results
Public health surveillance—the continuous collection and analysis of health data for evaluating and planning public health programs—can flag emerging epidemics and assess interventions.11
Two surveillance styles exist: passive and active. Through passive surveillance, health data are submitted voluntarily by the public and the medical community, among others.12 For Lyme and other tickborne zoonoses, passive surveillance might involve collection of ticks from people or pets, or human disease reporting. This type of disease oversight is generally inexpensive and lean on time and labor investment.13,14
Active surveillance, on the other hand, is more costly and labor intensive because it involves coordinated efforts by public health officials to gather medical data.12-15 For tickborne diseases, this might mean collecting both free-living ticks from the environment and ticks from small hosts, and testing them for pathogens in the laboratory.
Passive and active tickborne disease surveillance programs can be coupled as follows: Passive tick surveillance might be employed broadly, for collection of ecologic data, for instance, and then active surveillance can be applied in areas where elevated risk was identified through passive study.
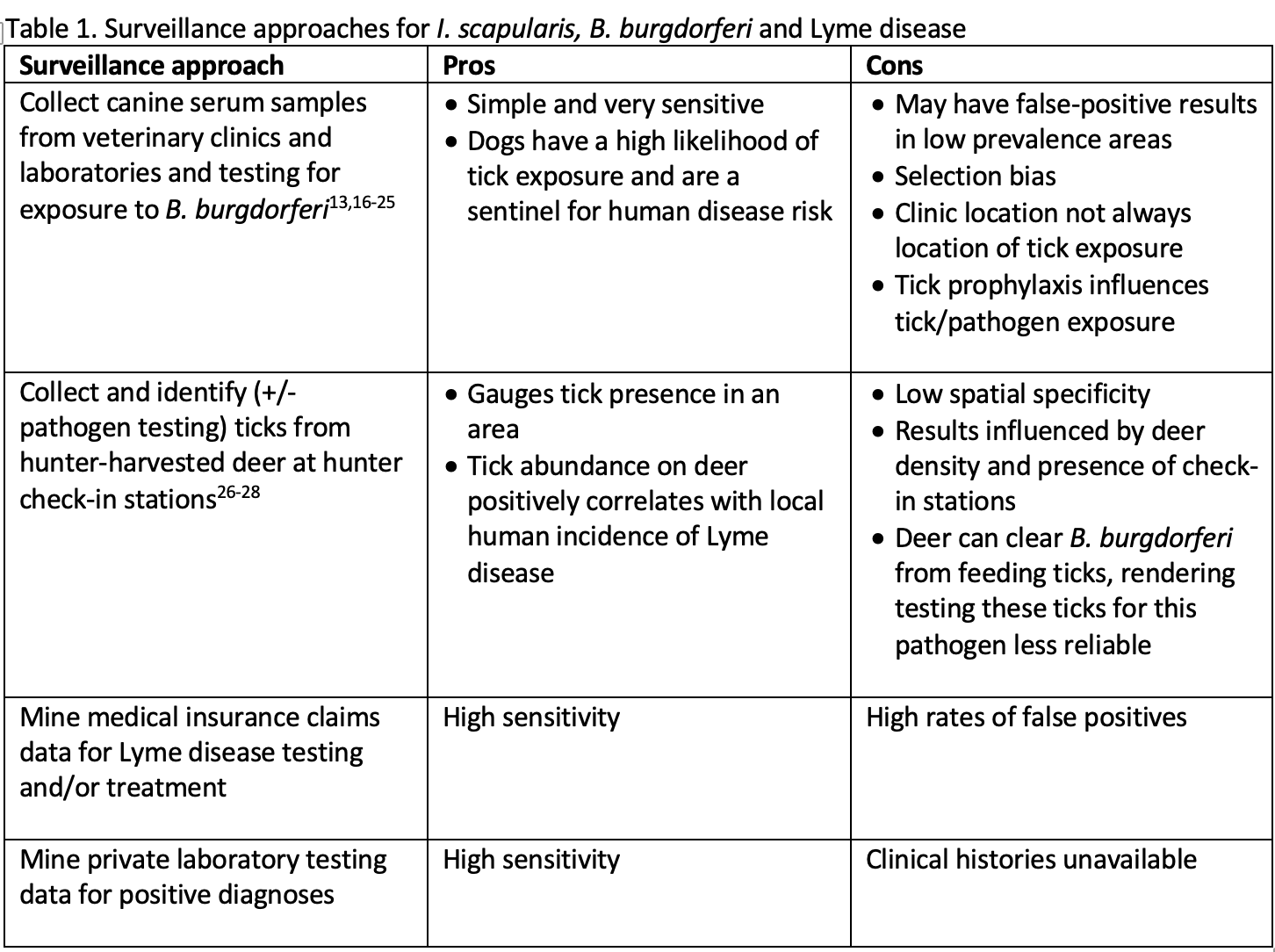
Table 1 outlines the variety of specific approaches for surveillance of I. scapularis, B. burgdorferi and Lyme disease that were tagged and analyzed.
Tickborne zoonoses surveillance programs have many challenges. During the early stages of emergence, low awareness can hinder reporting efforts by the public and the medical community.29-32 Furthermore, as growing awareness boosts reporting, the true incidence can falsely appear to be surging.33,34
Surveillance programs are further hampered by arbitrary geographical borders,35 nonstandardized data,32 communication issues impacting data collection and analysis,36,37 and insufficient resources for extending studies longitudinally.35
Finally, ticks themselves are tough to track. Their questing behavior and spot densities are variable and inconsistent.38,39 Plus, their bites can go undetected, and a delay exists between bite and symptom onset.31
Shortly after I. scapularis reared its ugly head and announced its spread through Canada in the early 1990s, public health officials and researchers met to address risks.40 Multi-province active and passive tick surveillance programs mapped tick densities, and public education programs were initiated.41-45 In some regions, passive tick surveillance programs gave way to targeted active surveillance aimed at detecting changes in ticks’ ranges.29,44-49
In 2009, Lyme disease was declared a reportable disease in Canada,50 and in 2014, the Canadian Parliament passed legislation mandating a national framework for Lyme disease surveillance.51,52
Novel surveillance approach
This comprehensive review of surveillance systems for emerging tick-borne diseases highlights the need to make them adaptive as vectors, hosts, pathogens and ecologic factors evolve.29,31,36,53-56 An adaptive approach that incorporates necessary experts,2,3,37,57 comprehensive data points,35,58 standardized measurements59 and resources sufficient for maintaining long-term research protocols33 can enhance surveillance data across all phases of disease emergence, from pre-emergence to endemicity.
Though this framework was created for tickborne zoonoses, it can be applied to a variety of other vector-borne and zoonotic diseases.60-62
Dr. Capuzzi is a small animal veterinarian and journalist based in the Philadelphia area.
References
1. Jones KKE, Patel NGN, Levy MA, et al. Global trends in emerging infectious diseases. Nature 2008;451:990-993.
2. Jones BA, Grace D, Kock R, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA 2012;110:8399-8404.
3. Karesh WB, Dobson A, Lloyd-Smith JO, et al. Ecology of zoonoses: natural and unnatural histories. Lancet 2012;380:1936-1945.
4. Mead PS, Epidemiology of Lyme borreliosis. Infect Dis Clin North Am 2015;29:187-201.
5. Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science 1993;260:1610-1616.
6. Gray JS. Risk assessment of Lyme borreliosis. Cent Eur J Med 1999;111:990-993.
7. Rizzoli A, Hauffe HC, Carpi G, et al. Lyme borreliosis in Europe. Eur Surveill 2011;16:1-8.
8. Ogden NH, Lindsay LR, Morshed M, et al. The rising challenge of Lyme borreliosis in Canada. Can Commun Dis Rep 2008;34:1-19.
9. Clow KM, Leighton PA, Pearl DL, et al. A framework for adaptive surveillance of emerging tick-borne zoonoses. One Health. 2019;7:100083.
hncbi.nlm.nih.gov/pmc/articles/PMC6376153/?report=classic
10. Pawson R, Greenhalgh T, Harvey G, et al. Realist review—a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34.
11. Public health surveillance. World Health Organization website:who.int/topics/public_health_surveillance/en/. Accessed March 13, 2020.
12. Nsubuga P, White M, Thacker S, et al. Public health surveillance: a tool for targeting and monitoring interventions. In: Jamison D, Breman J, Measham A, eds. Disease control priorities in developing countries. Washington DC: Oxford University Press; 2006:997-1015.
13. Johnson JL, Ginsberg HS, Zhioua E, et al. Passive tick surveillance dog seropositivity and incidence of human Lyme disease. Vector Borne Zoonotic Dis 2004;4:137-142.
14. Ogden NH, Koffi JK, Lindsay LR. Assessment of a screening test to identify Lyme disease risk. Can Commun Dis Rep 2014;40:1-5.
15. Nelder MP, Russell C, Lindsay LR, et al. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS ONE 2014;9:e105358.
16. Naleway A, Belongia E, Kazmierczak J, et al. Lyme disease incidence in Wisconsin: a comparison of state reported rates with rates from a population-based cohort. Am J Epidemiol 2002;155:1120-1127.
17. McHugh L. Effect of electronic laboratory reporting on the burden of Lyme disease surveillance—New Jersey, 2001-2006. MMWR Morb Mortal Wkly Rep 2008;57:42-45.
18. Braks M, van der Giessen J, Kretzschmar M, et al. Towards an integrated approach in surveillance of vector-borne diseases in Europe. Parasit Vectors 2011;4:192.
19. Diuk-Wasser MA, Hoen AG, Cislo P, et al. Human risk of infection with Borrelia burgdorferi the Lyme disease agent in eastern United States. Am J Trop Med Hyg 2012;86:320-327.
20. Nadolny RM, Feldman KA, Pagac B, et al. Review of the mid-Atlantic tick summit III: a model for regional information sharing. Ticks Tick Borne Dis 2015;6:435-438.
21. Bhide M, Travnicek M, Curlik J, et al. The importance of dogs in eco-epidemiology of Lyme borreliosis: a review. Vet Med Czech 2004;49:135-142.
22. Duncan AW, Correa MT, Levine JF, et al. The dog as a sentinel for human infection: prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonotic Dis 2005;5:101-109.
23. Rand PW, Lacombe EH, Dearborn R, et al. Passive surveillance in Maine, an area emergent for tick-borne diseases. J Med Entomol 2007;44:1118-1129.
24. Hamer SA, Tsao JI, Walker ED, et al. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am J Vet Med 2009;70:49-56.
25. Mead PS, Goel R, Kugeler KJ. Canine serology as adjunct to human Lyme surveillance. Emerg Infect Dis 2011;17:1710-1712.
26. Smith FD, Ballantyne R, Morgan ER, et al. Estimating Lyme disease risk using pet dogs as sentinels. Comp Immunol Microbiol Infect Dis 2012;35:163-167.
27. Millen K, Kugeler KJ, Hinckley AF, et al. Elevated Lyme disease seroprevalence among dogs in a nonendemic county: harbinger or artifact? Vector Borne Zoonotic Dis 2013;13:340-341.
28. Herrmann JA, Dahm NM, Ruiz MO, et al. Temporal and spatial distribution of tick-borne disease cases among humans and canines in Illinois (2000-2009). Environ Health Insights 2014;8:15-27.
29. Qurollo BA, Chandrashekar R, Hegarty BC, et al. A serological survey of tick-borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii and Borrelia burgdorferi species-specific peptides. Infect Ecol Epidemiol 2014;4:24699.
30. Savić S, Vidić B, Grgić Z, et al. Emerging vector-borne diseases—incidence through vectors. Front Public Health 2014;2:267.
31. Gill JS, McLean RG, Neitzel DF, et al. Serologic analysis of white-tailed deer sera for antibodies to Borrelia burgdorferi by enzyme-linked immunosorbent assay and western immunoblotting. J Clin Microbiol 1993;31:318-322.
32. Gill JS, McLean RG, Shriner RB, et al Serologic surveillance for the Lyme disease spirochete Borrelia burgdorferi in Minnesota by using white-tailed deer as sentinel animals. J Clin Microbiol 1994;32:444-451.
33. Riehle M, Paskewitz SM. Ixodes scapularis (Acari: Ixodidae): status and changes in prevalence and distribution in Wisconsin between 1981 and 1994 measured by deer surveillance. J Med Entomol 1996;33:933-938.
34. Daniels TJ, Fish D, Levine JF, et al. Canine exposure to Borrelia burgdorferi and prevalence of Ixodes dammini (Acari: Ixodidae) on deer as a measure of Lyme disease risk in the northeastern United States. J Med Entomol 1993;30:171-178.
35. Hinckley AF, Connally NP, Meek JI, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 2014;59:676-681.
36. Bacon R, Kugeler KJ, Mead PS. Surveillance for Lyme disease—United States, 1992–2006. MMWR Morb Mortal Wkly Rep 2008;57:1-9.
37. Smith R, O'Connell S, Palmer S. Lyme disease surveillance in England and Wales, 1986-1998. Emerg Infect Dis 2000;6:404-407.
38. Stefanoff P, Orlíková H, Príkazský V, et al. Cross-border surveillance differences: tick-borne encephalitis and Lyme borreliosis in the Czech Republic and Poland, 1999-2008. Cent Eur J Public Health 2014;22:54-59.
39. Hukic M, Numanovic F, Sisirak M, et al. Surveillance of wildlife zoonotic diseases in the Balkans region. Medicinski Glasnik 2010;7:96-105.
40. Prusinski MA, Kokas JE, Hukey KT, et al. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae) and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands. J Med Entomol 2014;51:226-236.
41. Canada Health. Consensus conference on Lyme disease. Can J Infect Dis 1991;2:49-54.
42. Ogden NH, Trudel L, Artsob H, et al. Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with Lyme Borreliosis agent Borrelia burgdorferi. J Med Entomol 2006;43:600-609.
43. Milord F, Nguon S. Le risque de la maladie de Lyme au Canada en relation avec les changements climatiques: évaluation des systèmes de surveillance. http://collections.banq.qc.ca/ark:/52327/1988807. Published March 2010. Accessed March 13, 2020.
44. Lyme Disease in Newfoundland. health.gov.nl.ca/health/lyme_disease.html. Accessed March 13, 2020.
45. Alberta health. Tick surveillance 2014 summary. Government of Alberta website: https://open.alberta.ca/dataset/f0b7698f-03d4-4d32-858f-141ec7c3c108/resource/d4ef2edb-2421-4be2-a4dc-cfca183c67d6/download/tick-surveillance-summary-2014.pdf. Published April 2015. Accessed March 13, 2020.
46. Lyme disease fact sheet. Government of Saskatchewan website: saskatchewan.ca/residents/health/diseases-and-conditions/lyme-disease. Updated May 2018. Accessed March 13, 2020.
47. Ontario Agency for Health Protection and Promotion (Public Health Ontario). Technical report: Update on Lyme disease prevention and control, 2nd ed. Toronto, ON: Queen’s Printer for Ontario; 2016. Available at: publichealthontario.ca/-/media/documents/lyme-disease-prevention-technical.pdf?la=en.
48. Lyme disease: A report on Lyme disease epidemiology and surveillance in Nova Scotia. Government of Nova Scotia website: novascotia.ca/dhw/populationhealth/documents/Lyme-Disease-Epidemiology-and-Surveillance-in-Nova-Scotia.pdf Published April 2012. Accessed March 13, 2020.
49. Surveillance map: blacklegged tick risk areas in Manitoba. Government of Manitoba website: https://www.gov.mb.ca/health/publichealth/cdc/tickborne/surveillance.html.Published March 15, 2018. Accessed March 13, 2020.
50. Gabriele-Rivet V, Arsenault J, Badcock J, et al. Different ecological niches for ticks of public health significance in Canada. PLoS ONE 2015;10:e0131282.
51. National Lyme disease surveillance in Canada, 2009–2012. Government of Canada website: healthycanadians.gc.ca/publications/diseases-conditions-maladies-affections/2009-2012-lyme/index-eng.php. Modified February 9, 2015. Accessed March 13, 2020.
52. Laupland K, Valiquette L. Bill C-442: shining the limelight on the Lyme-like? Can J Infect Dis Med Microbiol 2014;25:239-240.
53. Parliament of Canada Private member's Bill - Bill C-442. 2014. Parliament of Canada website: parl.ca/DocumentViewer/en/41-2/bill/C-442/royal-assent/page-ToC. Accessed March 13, 2020.
54. Semenza JC. Prototype early warning systems for vector-borne diseases in Europe. Int J Environ Res Public Health 2016;12:6333-6351.
55. Kulkarni MA, Berrang-Ford L, Buck PA, et al. Major emerging vector-borne zoonotic diseases of public health importance in Canada. Emerg Microbes Infect 2015;4:e33.
56. Wardrop NA. Integrated epidemiology for vector-borne zoonoses. Trans R Soc Trop Med Hyg 2016;110:87-89.
57. Spielman A, Levine JF, Wilson ML. Vectorial capacity of north American Ixodes ticks. Yale J Biol Med 1984;57:507-513.
58. Dumler JS, Bakken JS. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis 1995;20:1102-1110.
59. Dibernardo A, Cote T, Ogden NH, et al. The prevalence of Borrelia miyamotoi infection and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasit Vectors 2014;7:183-190.
60. Funk S, Bogich TL, Jones KE, et al. Quantifying trends in disease impact to produce a consistent and reproducible definition of an emerging infectious disease. PLoS ONE 2013;8:e69951.
61. Smith KF, Dobson AP, McKenzie FE, et al. Ecological theory to enhance infectious disease control and public health policy. Front Ecol Environ 2005;3:29-37.
62. Carver S, Kilpatrick AM, Kuenzi A, et al. Environmental monitoring to enhance comprehension and control of infectious diseases. J Environ Manag 2010;12:2048-2055.
