Toxicology Brief: The toxicity of iron, an essential element
Iron is the most abundant trace mineral in the body and is an essential element in most biological systems.
Iron is the most abundant trace mineral in the body and is an essential element in most biological systems.1,2 It is likely that iron was essential for developing aerobic life on Earth.3 But iron is toxic to cells in excessive amounts. Acute iron poisoning is common and potentially lethal in dogs, cats, and many other animals. Iron is also a leading cause of unintentional poisoning deaths in children less than 6 years old.
Normal iron content and storage
About 70% of the iron in mammals is found in hemoglobin, and about 5% to 10% is found in myoglobin. When bound to normal hemoglobin and myoglobin, iron is in the ferrous (Fe2+ ) form.1,2,4 Up to 25% of iron in the body is in the ferric (Fe3+ ) form and is stored in hemosiderin, ferritin, and transferrin in the liver, spleen, and bone marrow.1,2,5 Ferric iron is used in iron-containing enzymes, such as peroxidase, catalase, and cytochrome-c.
Sources
One reason iron toxicosis is such an important problem is that the general public is often unaware of the potential toxicity of products that are considered natural and necessary for our health.6
Another reason is that many pharmaceutical preparations contain iron. Multivitamins containing iron are readily available. Many are brightly colored and sugarcoated, making them attractive to animals and small children. In addition, several iron supplements are available over the counter. Another frequent source of iron overdose in pets is prenatal vitamins. Many prescription prenatal vitamins contain more than 60 mg of elemental iron in each pill, so animals can develop severe iron toxicosis even if only a few tablets are ingested.
Numerous other products contain iron, including one-time-use heating pads. Iron can also be found in fertilizers and pesticides and in the soil.1,2,4
Iron is also used in injectable products and is bound to proteins in supplements (chelated iron) to treat iron deficiencies in animals. There are several forms of injectable iron (iron dextran, iron dextrin, iron sorbitol, ferric ammonium citrate) and several chelated forms of iron. Chelated iron is almost as effective in treating iron deficiencies as other salts are but is about a fourth as toxic.1,2,4 Product labels do not always indicate if the iron is chelated. Most products that contain iron have it in a salt form. Table 1 lists several iron salts and the percentage of elemental iron in each.
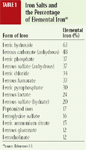
Table 1. Iron Salts and the Percentage of Elemental Iron*
Iron absorption
Iron absorption is a two-step process. First, iron ions are absorbed from the intestinal lumen into mucosal cells. Ferrous iron is better absorbed than ferric iron because ferric iron precipitates out of solution at around pH 7 or under normal physiologic conditions.7 However, both forms can be absorbed if they are ionized.1,2,5 Because iron must be ionized to be absorbed, metallic iron and iron oxide (rust) are not generally of concern when they are ingested.1,2 Most iron absorption occurs in the duodenum and upper jejunum, but in animals with iron toxicosis, the iron seems to be well-absorbed along all parts of the intestinal tract.1,2,5,6 A diet high in sugar and vitamin C increases iron absorption, while a high-phosphate diet reduces iron absorption.1,2,4,5 But in acute overdoses, the iron seems to be absorbed in a passive, concentration-dependent fashion, similar to how most other metals are absorbed.
Second, iron is transferred to ferritin or into circulation bound to transferrin proteins. Transferrin is an alpha1-globulin produced in the liver.1,2,7 Complexed with transferrin, iron is distributed to other iron storage locations in the body. A unique feature of iron metabolism is the almost complete absence of iron excretion. Any iron lost from hemoglobin degradation is rapidly bound to transferrin and transported to the bone marrow for the resynthesis of hemoglobin.2,7 Consequently, little iron is lost in the urine and feces. In addition, iron loss is not notably increased even after iron overdoses.2,4 Most iron loss is through the exfoliation of gastrointestinal mucosal cells in all mammals and through menstrual blood loss.5 While anywhere from 2% to 15% of the iron ingested is absorbed, only about 0.01% of the iron body burden is eliminated every day.1,5
Mechanism of action
When the absorbed iron is not bound to protein, it produces a variety of harmful free radicals. Consequently, the concentration of iron is rigorously controlled in mammalian cells and biological fluids. Acute iron toxicosis causes both a direct corrosive effect on the gastrointestinal tract and cellular damage due to circulating unbound iron.2 Large doses of iron may overcome the rate-limiting absorption step and allow excessive iron to enter the body. When iron-binding proteins become saturated, free iron ions are allowed into the general circulation.2,4-6 Free iron penetrates the cells of the liver, heart, and brain. At the cellular level, free iron increases lipid peroxidation with resulting membrane damage to mitochondria, microsomes, and other cellular organelles.1
Iron exerts its most profound effects on the cardiovascular system. Excessive iron can cause fatty necrosis of the myocardium, postarteriolar dilatation, increased capillary permeability, and reduced cardiac output.2 Free iron stimulates serotonin and histamine release as well as systemic metabolic acidosis caused by lactic acid accumulation. All these mechanisms lead to shock. Excessive iron also interferes with clotting mechanisms, augmenting hemorrhagic processes.1,2,4 Excessive iron also has been reported to cause thrombocytopenia.5
Excessive iron causes metabolic acidosis through several mechanisms. First, lactic acidosis occurs because of hypovolemia and hypotension. Iron disrupts oxidative phosphorylation by interfering in the electron transport chain. Thus, anaerobic metabolism is promoted. As ferrous iron is converted to ferric iron, hydrogen ions are released, adding to the metabolic acidosis. Free iron ions also inhibit the Krebs cycle, and organic acids accumulate.5
The liver accumulates free iron in Kupffer cells and the hepatocytes. The iron localizes in mitochondria of these cells and damages several cell organelles.5 Eventually, hypoglycemia, hyperammonemia, coagulation defects, and hepatic encephalopathy occur.2,5 Free iron inhibits the thrombin-induced conversion of fibrinogen to fibrin. Histopathologic evidence of iron-induced hepatic damage includes cloudy and swollen hepatocytes, portal iron deposition, fatty metamorphosis, and massive periportal necrosis.2,4,5
Toxicity
Since no mechanism exists for excreting iron, toxicity depends on the amount of iron already in the body. Consequently, some animals develop clinical signs of toxicosis even when they receive doses that cause no problems in other animals. Iron is most toxic when given intravenously. Intramuscular injections are less toxic, and iron given orally is the least toxic, probably because the amount of iron absorbed orally is not 100% of the dose ingested.4 When assessing the potential toxicity of an iron overdose, the amount of elemental iron in the products ingested must be determined (Table 1).4 For example, if a 500-mg tablet of ferrous gluconate was ingested, only 60 mg of elemental iron would have been ingested (500 mg X 0.12).
No clinical signs of toxicosis are expected in dogs ingesting less than 20 mg/kg of elemental iron. Dogs ingesting between 20 and 60 mg/kg of elemental iron can develop mild clinical signs. When the amount of elemental iron ingested is greater than 60 mg/kg, serious clinical signs can develop.2 In all animals, oral doses between 100 and 200 mg/kg are potentially lethal.2,4
Clinical signs
Iron toxicosis manifests clinically in four stages. The first stage occurs in the six hours after an iron overdose. It is marked primarily by gastrointestinal effects, such as vomiting, diarrhea, and gastrointestinal bleeding.2,4-6 The greatest mucosal damage occurs on an empty stomach. Most animals with mild to moderate iron toxicosis do not progress beyond this stage.5
The second stage occurs six to 24 hours after the overdose. This is referred to as a latent period, a period of apparent clinical recovery. In animals with severe iron toxicosis, this recovery period is transient and soon progresses to the third stage.2
The third stage of iron toxicosis occurs about 12 to 96 hours after the initial clinical signs develop. This stage is marked by lethargy, a recurrence of gastrointestinal signs, metabolic acidosis, shock, hypotension, tachycardia, cardiovascular collapse, coagulation deficits, hepatic necrosis, and possibly death.2,5,6
The fourth stage, which may occur two to six weeks after the iron overdose,2,5 is when animals that had gastrointestinal ulcerations and survived are healing. As these ulcerations heal, scarring occurs and strictures may develop. Even animals that had only gastrointestinal irritation in the first stage of iron toxicosis are at risk of developing strictures.2
Other abnormalities noted when iron overdoses occur are dehydration, hypovolemia, anemia, evidence of hepatic necrosis (elevated alanine transaminase and aspartate transaminase activities), and liver failure (hypoglycemia, hyperammonemia).2,5 In addition, iron toxicosis causes coagulation disturbances that are related to thrombocytopenia, hypoprothrombinemia, and impaired clotting factor synthesis.5 In people, the presence of hyperglycemia and leukocytosis often indicates a serum iron concentration of greater than 30 ug/dl.2 Finally, iron toxicosis results in several central nervous system signs. Often these signs result from effects on other cellular processes. For example, metabolic acidosis and hepatotoxicity can lead to other signs such as lethargy and hepatic encephalopathy.5 Other central nervous system signs that occur are comas, seizures, and tremors.1,2,5
Diagnosis
Testing an animal's serum iron concentration is the best method to confirm iron poisoning. It is also beneficial to measure total iron-binding capacity, although neither test alone is sufficient to determine whether treatment is needed. Most human hospitals offer serum iron concentration and total iron-binding capacity testing, but not all veterinary clinical pathology laboratories do.
Since the normal serum iron concentration and normal total iron-binding capacity can vary from animal to animal, it is best to measure both and correlate the test results with clinical observations. Serum iron concentrations can change dramatically during the first few hours after ingestion, so repeat the serum iron test four to six hours after initial measurement. When the serum iron concentration exceeds the total iron-binding capacity or the serum iron concentration is greater than 500 ug/dl, severe systemic effects can be expected. Normal serum iron-binding capacity is usually about 25% to 30% saturated.2 Every laboratory is different, but an example of how the serum iron concentration and total iron-binding capacity results are reported is Fe = 134 ug/dl and total iron-binding capacity = 436 ug/dl, or 134/436 X 100 = 30.7% saturated.
Obtaining multiple blood samples to test serum iron concentrations may be indicated, especially when the total iron dose is unknown or the animal is symptomatic. An abdominal radiographic examination can be useful to identify metallic objects since iron tablets are radiopaque.2,5
Treatment
A protocol for treating iron toxicosis is described in Figure 1. Animals that have recently ingested large doses of iron will benefit from gastrointestinal decontamination. In animals that can vomit, induce emesis with 3% hydrogen peroxide (1 to 5 ml/kg orally), apomorphine hydrochloride (0.03 mg/kg intravenously, 0.04 mg/kg intramuscularly), or other appropriate emetics.8 Gastric lavage can be performed on anesthetized animals, although it may not be effective if large pills are involved or if the pills adhere to gastric mucosa. Place a cuffed endotracheal tube to prevent aspiration of lavage material.2 In a recent study, activated charcoal adsorbed ferrous sulfate solution at a pH environment consistent with that of the duodenum.9 It has been suggested that iron can be precipitated to a nonabsorbable form in the digestive tract by using sodium phosphate, sodium bicarbonate, or magnesium hydroxide; however, the clinical significance of this therapy is questionable.2,4,6

Figure 1. Management of Iron Toxicosis
Restoring fluids, electrolytes, and acid-base balance is essential to successfully treating iron toxicosis. Fluids are also needed to prevent hypovolemic shock. Administer fluids based on the animal's maintenance and replacement needs.2 Monitor electrolytes, and correct any abnormalities. Administering gastrointestinal protectants such as sucralfate, cimetidine, misoprostol, or other inhibitors of gastric acid secretion may also be helpful.2,10
Chelation therapy is indicated in animals at risk of or showing clinical signs of severe iron toxicosis. This includes animals that ingest more than 60 mg/kg of elemental iron, animals that have a total iron-binding capacity that is greater than the serum iron concentration, or animals that have a serum iron concentration greater than 500 ug/dl. Deferoxamine mesylate (Desferal—Novartis Pharmaceuticals), the chelator of choice for excessive iron in the body, is the only chelator available that seems to be effective at reducing serum iron concentrations. The recommended dosage of deferoxamine is 40 mg/kg given intramuscularly every four to eight hours. Alternatively, give deferoxamine as a continuous infusion at the rate of 15 mg/kg/hr. Continue chelation therapy until the serum iron concentrations decrease below 300 ug/dl and the clinical signs resolve. Often, iron toxicosis requires two or three days of chelation therapy.2,4,5 Deferoxamine causes reddish-colored urine, which indicates free iron is being excreted. In people, deferoxamine therapy is continued until the urine color returns to normal.6 Deferoxamine has not been reported to cause iron deficiency.
Calcium EDTA has also been used to reduce serum iron concentrations but has not been shown to reduce mortality in cases of acute iron poisoning. An experimental iron chelator, N, N'-bis(2-hydroxybenzyl) ethylenediamine-N, N'-diacetic acid monosodium salt (NaHBED), has been used to successfully treat iron overdoses in dogs and monkeys and was shown to be about twice as effective as deferoxamine and with fewer side effects.11 If NaHBED is approved for use in people, it may also become an alternative iron chelator for animals.
Monitoring and prognosis
Monitor all treated animals for four to six weeks for evidence of gastrointestinal obstruction.2 Once signs of iron toxicosis have developed, the prognosis is guarded.
Severe iron poisoning requires a lot of time and effort to treat effectively. Thus, treatment can become costly. In addition, it is often difficult to obtain deferoxamine. If the serum iron concentration exceeds 500 ug/dl and a chelator is unavailable, the prognosis is poor.
Prevention is the best treatment for iron toxicosis. Teaching owners about the dangers of iron toxicosis and the importance of keeping all medications, multivitamins, and iron supplements out of reach of animals will help avoid serious injury.
REFERENCES
1. Goyer RA. Toxic effects of metals. In: Klaassen CD, ed. Casarett & Doull's toxicology: the basic science of poisons. 5th ed. New York City, NY: McGraw-Hill, 1996;715-716.
2. Greentree WF, Hall JO. Iron toxicosis. In: Bonagura JD, ed. Kirk's current therapy XII small animal practice. Philadelphia, Pa: WB Saunders Co, 1995;240-242.
3. Williams RJ. Biomineralization: iron and the origins of life. Nature 1990;343:213-214.
4. Osweiler GD, Carson TL, Buck WB, et al. Iron. In: Clinical and diagnostic veterinary toxicology. 3rd ed. Dubuque, Iowa: Kendall/Hunt Publishing Co, 1985;104-106.
5. Hillman RS. Hematopoietic agents: growth factors, minerals, and vitamins. In: Hardman JG, Limbird LE, Molinoff PB, et al, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 9th ed. New York City, NY: McGraw-Hill, 1995;1311-1340.
6. Liebelt EL. Iron. In: Haddad LM, Shannon MW, Winchester JF, eds. Clinical management of poisoning and drug overdose. 3rd ed. Philadelphia, Pa: WB Saunders Co, 1998;757-766.
7. Ponka P, Schulman HM, Woodworth RC. Iron transport and storage. Boca Raton, Fla: CRC Press, 1990.
8. Dorman DC. Emergency treatment of toxicoses. In: Bongura JD, ed. Kirk's current veterinary therapy XII small animal practice. Philadelphia, Pa: WB Saunders Co, 1995;211-217.
9. Chyka PA, Butler AY, Herman MI. Ferrous sulfate adsorption by activated charcoal. Vet Hum Toxicol 2001;43:11-13.
10. Plumb DC. Veterinary Drug Handbook. 3rd ed. Ames: Iowa State University Press, 1999.
11. Bergeron RJ, Wiegand J, Brittenham, GM. HBED ligand: preclinical studies of a potential alternative to deferoxamine for treatment of chronic iron overload and acute iron poisoning. Blood 2002;99:3019-3026.
"Toxicology Brief" was contributed by Jay Albretsen, DVM, PhD, DABT, DABVT, Covance Laboratories, 3301 Kinsman Blvd., Madison, WI 53704. The department editor is Petra A. Volmer, DVM, MS, DABVT, DABT, College of Veterinary Medicine, University of Illinois, Urbana, IL 61802.