The definition of glaucoma is changing rapidly as our understanding of the pathogenesis of damage to the retina and optic nerve improves. However, the disease can still be described generally as an elevation in intraocular pressure (IOP) that is incompatible with normal ocular function. IOP is the result of a balance between production and drainage of aqueous humor. In clinical practice, glaucoma is caused by drainage disturbances, while cases of increased production are not recognized.
Production and drainage of aqueous humor
Aqueous humor is transparent fluid produced in the ciliary processes, from where it flows into the posterior chamber and through the pupil into the anterior chamber. After circulating in the anterior chamber and supplying the metabolic requirements of the lens and cornea, this fluid exits the eye through the iridocorneal angle (between the cornea and iris), which is spanned by pectinate ligaments. The drainage continues through the uveal and corneoscleral meshworks, eventually exiting the eye into the systemic venous circulation.
Aqueous humor may also exit the eye through an unconventional path, in which it diffuses through the iris and ciliary body (or through the vitreous) into the suprachoroidal space; from there, the fluid drains into the venous circulation. The importance of this route varies among species, accounting for 15% of the total drainage in dogs and 33% in horses, but only 3% in humans.
Classifying glaucoma
Glaucoma may be classified in one of two ways:
- Based on the cause, it can be classified as primary, where outflow problems are linked to genetic abnormalities in the drainage pathway, or secondary, where another ocular disease (e.g. lens luxation, uveitis) decreases outflow.
- Alternatively, glaucoma can be classified according to the state of the drainage angle. The angle may be open (in which case the obstruction is further downstream), narrow or closed.
These classifications are not mere semantics; rather, they have significant clinical implications. It is possible that secondary glaucoma may be cured if the primary ocular disease is treated successfully (e.g. surgical removal of a luxated lens). More important, if unilateral glaucoma is deemed secondary, it means the contralateral eye is not necessarily at risk.
On the other hand, dogs with primary glaucoma will require lifelong treatment. Moreover, in patients presenting with unilateral primary glaucoma, the contralateral eye is highly likely to develop the disease (on average within 8 months). In some breeds, examination of the angle will help determine whether the eye is at risk for developing primary glaucoma.
The two classification methods complement each other; in dogs, it is possible to encounter any of the possible glaucoma combinations: primary open angle, primary narrow angle, primary closed angle, secondary open angle and secondary closed angle glaucoma.
Primary glaucoma is extremely rare in cats, and for all practical purposes feline glaucoma is invariably a secondary disease.
Primary open angle glaucoma
Primary open angle glaucoma (POAG) is an inherited disease that has been investigated extensively in beagles, in which it was shown to be an autosomal recessive disorder. However, POAG has also been documented in keeshonds, Norwegian elkhounds, poodles and other breeds, although the mode of inheritance has not been established in these breeds.
As the name implies, the angle and pectinate ligaments in dogs with POAG are normal. It is assumed that the outflow obstruction, located in the uveal and corneoscleral meshworks, is the result of biochemical changes to the basement membrane in these regions. The disease is chronic in nature, with IOP increasing slowly over many months or years. Although the dog may present with buphthalmos or even with secondary lens luxation, vision frequently is retained in advanced stages of this disease.
Primary narrow and closed angle glaucoma (goniodysgenesis)
Primary narrow angle glaucoma is an inherited disease in American and English cocker spaniels; flat-coated, Labrador and golden retriever breeds; as well as basset hounds, Samoyeds, chow chows, Great Danes, Siberian huskies and others. A developmental abnormality results in the formation of dysplastic pectinate ligaments, which can be seen as sheets of tissue spanning the drainage angle.
During the first years of life, aqueous humor exits the eye through flow holes in the sheets, but eventually the flow holes fail to control aqueous fluid outflow adequately, resulting in elevation in the IOP. Most canine patients present with an acute attack of glaucoma, including congestion, edema, fixed dilated pupils and loss of sight. Although only one eye may be affected initially, both eyes should be evaluated and prophylactic treatment of the unaffected eye is advisable. Individual attacks in affected eyes may be treated successfully, but progressive angle narrowing and closure may develop. In these patients, the long-term prognosis for vision is extremely guarded.
Secondary glaucoma
In patients with secondary glaucoma, pressure rises from obstruction of aqueous humor outflow caused by another ocular disease, most commonly one of the following:
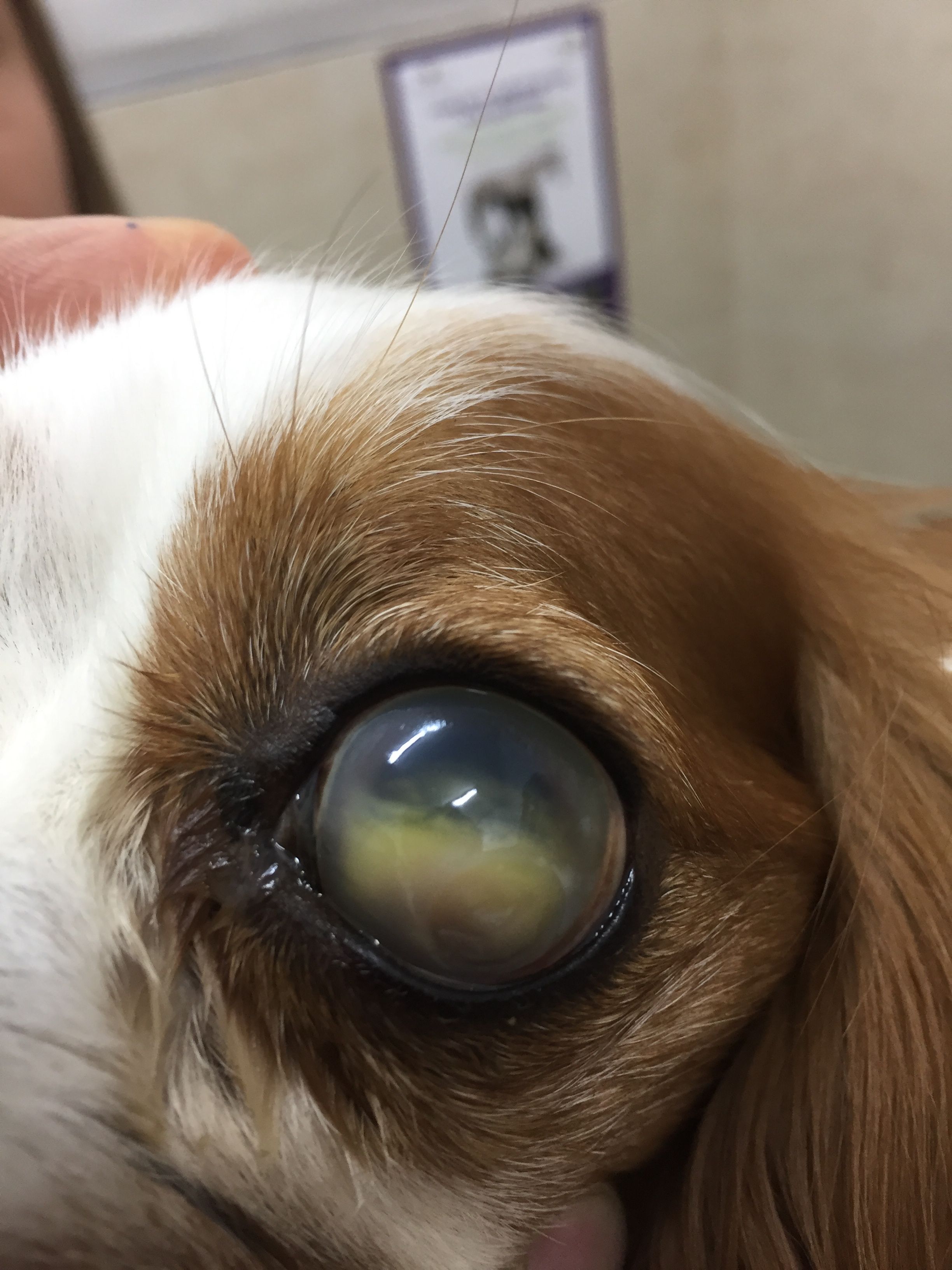
- Lens luxation. Because the lens serves as a barrier against forward movement of vitreous, any lens luxation (whether anterior or posterior) may allow vitreous humor to move into the anterior chamber and obstruct outflow (Figure 1). If the lens luxation is anterior, fluid outflow will be further impeded by the physical presence of the lens in the anterior chamber.
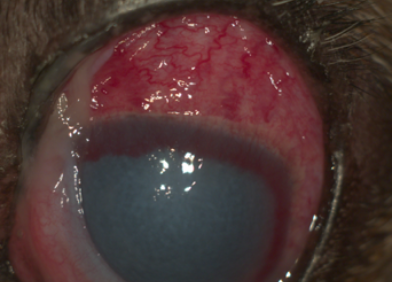
- Uveitis. In these patients, the inflammatory material in the anterior chamber (e.g. cells, platelets, fibrin) can obstruct the iridocorneal angle. In addition, uveitis can lead to adhesion between the iris and the lens or cornea (posterior and anterior synechia, respectively), further disrupting aqueous humor outflow (Figure 2).
- Intraocular tumor. Tumors can cause glaucoma by inducing uveitis. Furthermore, neoplastic cells can obstruct the angle, or the tumor (if large enough) can physically compress the angle. Intraocular tumors should be suspected in any senior patient with unilateral uveitis or unilateral glaucoma.
Guide to diagnosing glaucoma
Tonometry (measuring IOP)
In canine patients, the normal IOP range is 15 to 25 mm Hg. Elevation in IOP is defined as glaucoma, whereas low IOP is usually a sign of uveitis linked to increased unconventional outflow. The IOP measurement should be similar in both eyes. Differences of more than 10 mm Hg between eyes may be indicative of glaucoma. Likewise, normal pressure in an eye with uveitis is suspicious, as the hypotensive effect of uveitis may be masking elevation in pressure.
IOP cannot be measured digitally with one's fingers. It should be recorded by using a Schiotz (indentation) tonometer or, preferably, with a modern rebound or applanation tonometer.
Gonioscopy (examining the iridocorneal angle)
It is important to examine the angle to determine the risk of glaucoma in breeds with goniodysgenesis, or if the disease has already developed in the other eye. Gonioscopy is performed by a specialist using a special lens (i.e. goniolens) that is placed on the cornea. The lens refracts outgoing light and allows visualization of the entire angle to classify its state (Figure 3).
In dogs with unilateral glaucoma, the disease may be secondary; or it can be a primary disease, as one eye is frequently affected before the other. If unilateral primary glaucoma is suspected, referral for gonioscopy is mandatory so that prophylactic treatment can be initiated in the unaffected eye.
History and clinical signs
Canine glaucoma may affect all ocular layers and structures:
- Pain. Glaucoma is a painful disease. The pain can be expressed as blepharospasm or as general depression. While the pain may be more insidious in dogs with chronic glaucoma, many owners report a dramatic improvement in the animal's behavior following enucleation of a glaucomatous eye.
- Buphthalmos. Glaucoma may cause an increase in the size of the globe, resulting from stretching of the collagen fibers of the cornea and sclera. Buphthalmos usually occurs more frequently in patients with chronic disease as well as in young patients, as the sclera is more elastic and stretches more easily.
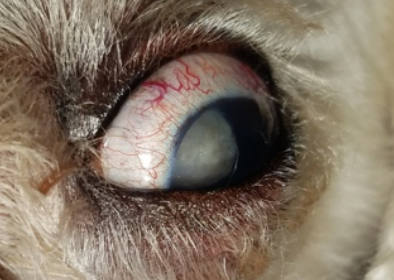
- Congestion of blood vessels. The eye will appear red from congestion of episcleral vessels (Figure 4).
- Corneal pathology. Elevated IOP damages the corneal endothelium, which is responsible for maintaining corneal dehydration, resulting in edema (see Figure 4). Stretching of the corneal fibers in patients with buphthalmos may cause rupture of the endothelial basement membrane. These ruptures, seen as white lines in the cornea, are called striate keratopathy and are pathognomonic for glaucoma.
- Pupils. In early stages of the disease, the pupil may be dilated slightly, and its response will be sluggish. In advanced or acute stages of the disease, the pupils are dilated and nonresponsive.
- Lens. The lens may luxate (or subluxate) from stretching and tearing of the zonules. As noted, the reverse may also be true, as luxated lens can cause glaucoma (see Figure 1).
- Retina, optic nerve and vision. Glaucoma will cause atrophy of the ganglion cell layer and other inner retinal layers. This atrophy is a result of local ischemia, caused by pressure on the retinal blood vessels (the outer retina is supplied by the choroid and is less affected by ischemia). Additional damage to the ganglion cells occurs from kinking of their axons as they exit the eye at the lamina cribrosa region. In this part of the eye, the effect of elevated IOP may be seen ophthalmoscopically as cupping of the optic disc. Because of the damage to the inner (and, eventually, outer) retina, the patient will suffer progressive or acute loss of vision, which may lead to complete blindness.
- End-stage glaucoma. As a result of chronic IOP elevation, the ciliary body may atrophy, causing decreased aqueous production, lowering of pressure and atrophy of the eye (phthisis bulbi).
Medical therapy
Osmotic diuretics
These drugs are not used for long-term treatment of glaucoma. Instead, they serve for emergency lowering of IOP in patients with acute attacks. The most commonly used drug in this category is intravenous mannitol administered slowly at 1 to 2 g/kg over 30 minutes; water is withheld for three to four hours.
Prostaglandin analogues
These drugs act by increasing the unconventional outflow. They are most effective in dogs because their effect is independent of the state of the angle (which is frequently blocked). These drugs are ineffective in cats because cats lack the receptor, and they are contraindicated in all patients with uveitis. Latanaprost, travaprost and other drugs in this category are administered once or twice a day.
Principles of glaucoma treatment in dogs
- The aims of glaucoma treatment are to prevent further vision loss and decrease the pain caused by IOP elevation. Currently, it is impossible to restore vision that has been lost due to glaucoma.
- Primary glaucoma requires lifelong treatment. The owner must understand that the disease can never be fully cured and the aim of therapy is to stabilize the IOP.
- Diagnosis of primary glaucoma in one eye mandates prophylactic treatment in the other eye. If you are uncertain, consider referring to a specialist for gonioscopy.
Carbonic anhydrase inhibitors
Carbonic anhydrase is a key enzyme in the production of aqueous humor and, therefore, its inhibition will result in lower production and decreased IOP. Just like prostaglandin analogues, this effect is independent of the state of the angle. The topical form of the drug (dorzolamide, brinzolamide) is administered twice a day and has none of the systemic side effects observed with the systemic inhibitors.
Topical miotics
These drugs increase drainage by opening the iridocorneal angle (through contraction of the iris and ciliary muscle). The most commonly used drug in this category is pilocarpine 1% to 4%, applied two to three times a day.
Beta-blockers
Sympatholytic drugs reduce aqueous production by reducing blood flow to the ciliary body. They are commonly used in humans, but their effectiveness in animals is controversial. Systemic side effects are common in small dogs, cats and animals with pulmonary or cardiovascular disease. Drugs in this category include timolol, levobunolol and betaxolol, which are administered once or twice daily. A preparation containing timolol and dorzolamide is commercially available and may be very efficacious.
Administering eye drop medications
No matter what ocular disease you are treating, one drop is always enough. The second drop simply washes out the first. If you apply two drops, you are wasting half the bottle and not gaining anything.
Hold the eye open—not shut—for 30 to 60 seconds after instilling the drops. Blinking increases drainage of tears and drugs from the eye, and decreases the contact time of the drug with the ocular surface.
Surgical treatment
Referral ophthalmology clinics may perform surgery to increase aqueous humor outflow (usually by implanting drainage tubes in the eye) or to decrease aqueous production through partial destruction of the ciliary body, using laser or cryotherapy.
However, frequently the (surgical or medical) treatment fails, and the practitioner is faced with a blind and painful eye. Patient welfare requires removal of this eye through enucleation. Owners of dogs with primary (but not secondary) glaucoma may be offered evisceration (i.e. implantation of a prosthesis in an empty scleral shell) to provide a more cosmetic appearance.
Dr. Ofri is professor of veterinary ophthalmology at The Robert H. Smith Faculty of Agriculture, Food & Environment, Koret School of Veterinary Medicine, The Hebrew University of Jerusalem. His special interest is retinal diseases, including glaucoma.