Developing a Vaccine for Lyme Disease
Recent advances such as chimeritope technology could potentially be used to develop vaccines against other tickborne diseases as well.

Growing cultures of B burgdorferi viewed with dark field microscopy.
Lyme disease is the most commonly reported vector-borne disease in humans in the United States.1 Its incidence in dogs has been increasing steadily due to expanding tick populations.2 In a webinar hosted by online continuing education provider VETgirl, Richard T. Marconi, PhD, talked about the technology his laboratory used to develop a new Lyme disease vaccine for dogs. This chimeric recombinant vaccine, Vanguard crLyme, is produced by Zoetis, the webinar sponsor.
“Lyme disease is a very serious illness, and prevention is very, very important,” said Dr. Marconi, a professor in the Department of Microbiology and Immunology at Virginia Commonwealth University Medical Center. Lyme vaccines and tick control products have not been sufficient to stop the spread of the disease, he added.
RELATED:
- CDC Warning Echoes Parasite Council's Predictions About Vector-borne Disease
- Tick Exposure and Kidney Disease Risk in Dogs
Dr. Marconi began by describing the morphology of Borrelia burgdorferi, the causative agent of Lyme disease. The mechanism of motility of these spiral-shaped bacteria, or spirochetes, is similar to that of a drill bit. Once a spirochete enters a mammal’s body, Dr. Marconi said, “that corkscrew motility allows the Lyme disease bacteria to penetrate quickly through tissue and disseminate to distal organs and tissue sites throughout the body.”
Treatment of Lyme disease can be difficult once the spirochetes have penetrated deep into tissues and organs, he said. Three to 4 weeks of antibiotic treatment are required to clear an infection; in some cases it may be necessary to repeat treatment. For this reason, coupled with the serious clinical consequences associated with Lyme disease, prevention is crucial.
How Spirochetes Adapt to Different Environments
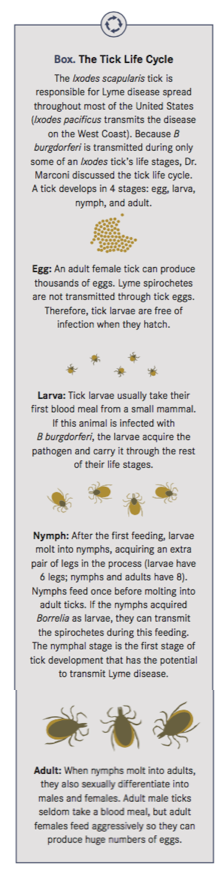
To be maintained in nature, the Lyme spirochetes must cycle between Ixodes ticks and mammals (enzootic cycle). Once spirochetes establish an infection in ticks, they reside for extended periods of time in the tick midgut, a harsh environment with few nutrients. When ticks ingest a blood meal, however, there
is a dramatic increase in available nutrients, which allows the spirochetes to ramp up their metabolic activity, divide and begin transit from ticks to mammals (Box).
However, this is a complicated process and spirochetes must be able to adapt rapidly to the radically different environments they encounter in the unfed tick, fed tick, and mammals. “One of the key things we need to know to develop an effective vaccine is exactly how the spirochetes adjust to these radically different environments,” Dr. Marconi said.
He used the analogy of human clothing to explain spirochete environmental adaptation. People wear different types of clothes in summer than in winter; that is, we change some of our surface properties to respond to changes in environmental conditions. Lyme spirochetes do the same thing, he said.
Outer Surface Proteins: Vaccine Targets
Outer surface proteins (Osps) “play important roles in the biology and adaptive responses of the Lyme disease spirochetes,” Dr. Marconi explained. They have also been at the center of Lyme disease research and vaccine development efforts. The 2 Osps that have received the most attention are OspA and OspC. These proteins are important in spirochete survival in ticks and in the transmission and infection process, respectively. The differential production of OspA and OspC is controlled by environmental conditions. OspA is a dominant protein on the surface of spirochetes residing in unfed ticks. However, as ticks feed, OspA production is turned off as OspC production is turned on. Upon entering a mammal, spirochetes are producing large amounts of OspC but no OspA.
OspA
Lyme disease vaccine development focused on OspA for many years, Dr. Marconi said. However, he added, the efficacy of OspA- targeting vaccines was approximately 72%. If a spirochete does not express OspA once it’s in a vaccinated animal, then how does a vaccine targeting OspA work at all? Dr. Marconi explained that the blood of an OspA-vaccinated mammal that has received sufficient booster immunizations contains anti-OspA antibodies. When a tick ingests this blood, the anti-OspA antibodies pass with the blood into the tick where they have the potential to kill spirochetes before they can transition to the production of OspC. “A transmission-blocking vaccine is a very attractive concept,” he remarked.
The problem, he continued, is α antibody levels in blood decline over time, eventually dropping below the threshold required to kill spirochetes inside the tick. Because the spirochetes that make it into a mammal lack OspA, they cannot be targeted by vaccine-induced antibody in the mammal. In addition, vaccines against OspA do not induce immune memory because a vaccinated animal’s memory cells are never exposed to OspA antigen during a natural infection.
OspC
“We realized that OspC had significant potential to help improve the Lyme disease vaccine,” Dr. Marconi said. Spirochetes express very low levels of OspC while they are in an unfed tick. After the tick takes a blood meal, OspC expression is upregulated dramatically in response to changes in temperature and pH. Spirochetes continue to express high levels of OspC after they have entered a mammal. A vaccine that targets OspC, therefore, can kill spirochetes inside a vaccinated animal. In addition, an OspC-based vaccine also has the potential to trigger immune memory.
Vaccine Development
Vaccines that target OspA are transmission-blocking vaccines: They prevent infectious organisms from entering the body. However, an animal must have a high antibody titer for an OspA vaccine to be protective. The function of OspC-targeting vaccines is to clear infection, and these vaccines also provide immune memory. Combining the 2 targets in a single vaccine should improve vaccine efficacy by eliciting protection through 2 synergistic mechanisms, Dr. Marconi said.
He discussed 2 general types of vaccines used in veterinary medicine: bacterins and subunit vaccines. He described bacterins as “bacterial soups.” Lyme disease bacterin vaccines are generated by growing the bacteria in the laboratory. The bacteria are then collected, inactivated, and homogenized to render them more amenable to injection. Bacterins contain extraneous ingredients that do not contribute to protective immune responses. “In the context of veterinary medicine, where you are delivering multiple vaccines during a single visit, it would really be preferable to have vaccines that are of higher purity” and have fewer unnecessary ingredients, he said.
Subunit vaccines consist of 1 or more highly purified recombinant proteins that are produced in a laboratory under controlled conditions, Dr. Marconi said. His group’s aim was to create a combination OspA and OspC subunit vaccine.
Developing the OspA component was simple, he said, because this protein does not vary substantially among Lyme spirochetes. In other words, a single version of OspA in a vaccine will induce antibodies that are effective against many strains of B burgdorferi.
OspC, on the other hand, has about 30 different variants. Most are designated with letters: OspC type A, OspC type B, and so forth. OspC variants are all the same protein, Dr. Marconi said, but their amino acid configurations differ. “Understanding the variation and diversity in this protein turned out to be critical in figuring out how to design a vaccine that could kill all the different strains that exist in nature,” he noted.
Dr. Marconi’s team found that dogs with positive Lyme antibody tests all had anti-OspC antibodies, but their antibodies were to different variants of the protein. Their next step was to isolate the specific parts of the OspC protein that induce antibody production. The parts of a protein that elicit anti- body production are referred to as epitopes. “We reasoned that if we could identify the epitopes of OspC that we could then utilize those defined portions of OspC to make a focused, refined, and broadly protective vaccine,” he said.
Dr. Marconi’s group ran immunoblot analyses on OspC subfragments and located 2 epitopes that induce antibody formation. These epitopes became the basis of the new vaccine.
Chimeritope Vaccine Technology
“The process that we applied is a new technology that we refer to as chimeritope technology,” Dr. Marconi said. The word chimeritope is a portmanteau meaning chimeric epitope-based protein. “Chimeric indicates that we are joining things together and what we are joining together are OspC epitopes derived from many different strains. Chimeritopes are desirable vaccine anti- gens because they contain only the immunologically relevant parts of many different OspC type proteins,” he said. Dr. Marconi explained that a protein constructed this way “can trigger the formation of [antibodies] to the diverse OspC type proteins that exist in nature and, therefore, provide broad protection.”
Further testing showed that a vaccine made with the OspC chimeritope induced the production of antibodies that could kill B burgdorferi. The spirochete elimination rate in the laboratory was higher with an experimental combination vaccine that contained both OspA and the chimeric OspC. Results from efficacy studies showed that compared with placebo and single-component vaccines, the combination vaccine significantly reduced lesions in the skin and joints of vaccinated dogs exposed to ticks carrying B burgdorferi, Dr. Marconi said.
Tests of the chimeritope vaccine that was eventually brought to market confirmed that the vaccine prevented infection, protected dogs against skin and joint lesions after exposure to ticks carrying B burgdorferi, and caused no major adverse effects. The most common immediate postvaccination reaction (<30 minutes after vaccination) was vocalization upon injection, occurring in 0.73% of dogs. The most common late postvaccination reaction (>30 minutes to 14 days after vaccination) was injection-site edema, which occurred in 3.81% of dogs.3
Vanguard crLyme received approval from the Food and Drug Administration in January 2016, said Dr. Marconi. It is administered by subcutaneous injection. Dogs should receive an initial series of 2 doses given 3 weeks apart. A 15-month duration of immunity has been established for Vanguard crLyme.
Dr. Walden received her doctorate in veterinary medi- cine from North Carolina State University in 1994. After an internship at Auburn University College of Veterinary Medicine, she returned to North Carolina, where she has been in companion animal general practice for over 20 years. Dr. Walden is also a board-certified editor in the life sciences and owner of Walden Medical Writing.
References:
- Lyme disease: data and statistics. Centers for Disease Control and Prevention website. cdc.gov/lyme/stats/. Updated November 13, 2017. Accessed April 29, 2018.
- Littman MP, Gerber B, Goldstein RE, Labato MA, Lappin MR, Moore GE. ACVIM consensus update on Lyme borreliosis in dogs and cats [published online March 22, 2018]. J Vet Intern Med. doi: 10.1111/jvim.15085.
- Zoetis. Vanguard crLyme efficacy study. zoetisus.com/products/dogs/vanguard-crlyme/resources/vanguard-crlyme-efficacy-information.pdf. Published December 2015. Accessed April 27, 2018.
