FDA OKs cell therapy clinical trial for feline gingivostomatitis
The investigational trial will evaluate the safety, efficacy, and potency of using stem cells to treat cats with refractory gingivostomatitis.
Cats with gingivostomatitis may soon find relief. (Image courtesy VetCell Therapeutics USA)
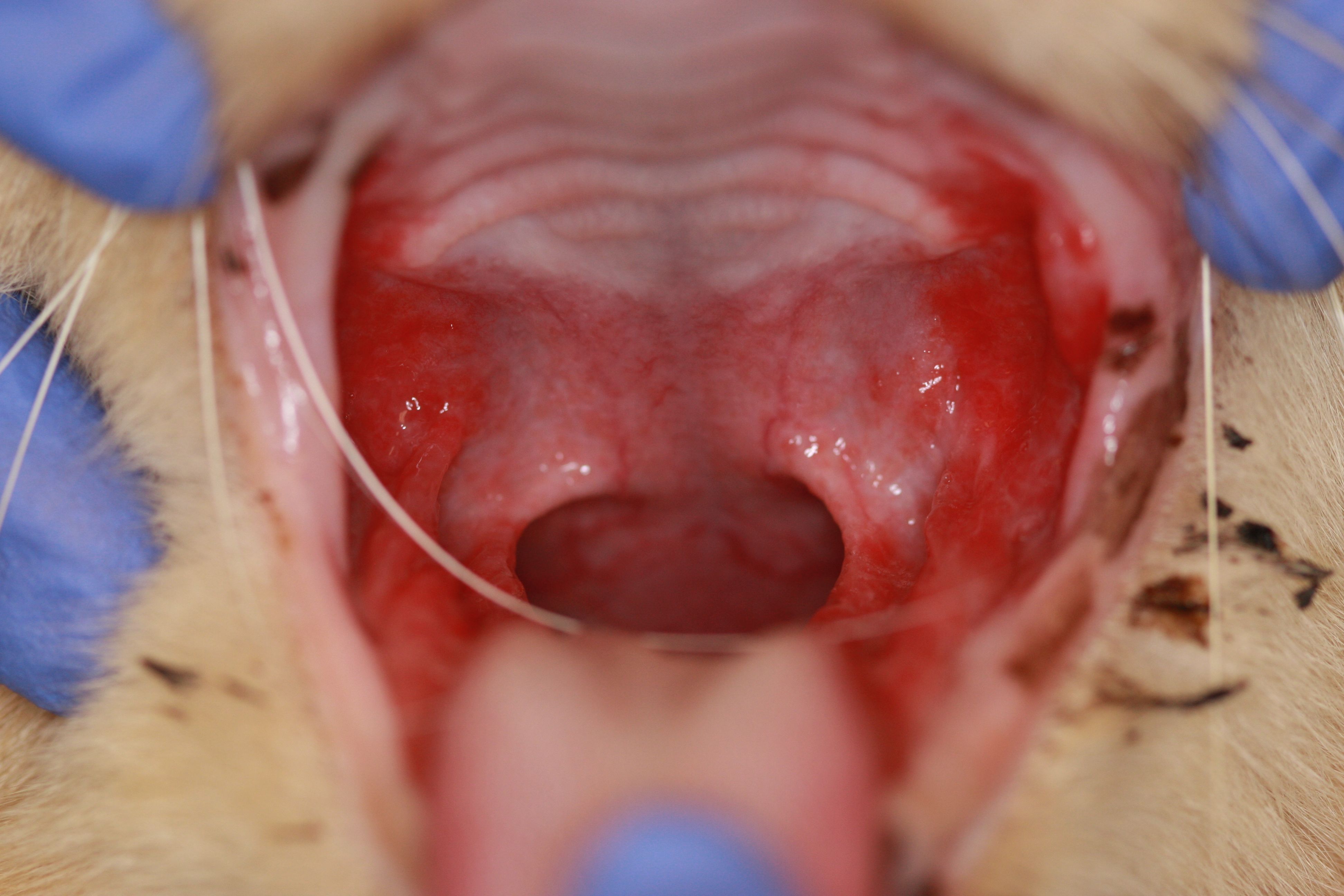
There may be good news for cats suffering from gingivostomatitis, a painful dental disease marked by severe, chronic inflammation of the gingiva and mucosa. VetCell Therapeutics USA has received FDA approval to begin a clinical trial with its new cell therapy, DentaHeal. Developed at the University of California, Davis School of Veterinary Medicine, Dentaheal is an allogeneic, adipose-derived, mesenchymal stem cell therapy administered intravenously that aims to correct immune abnormalities linked to feline chronic gingivostomatitis (FCGS).
The nationwide trial will include 200 cats with FCGS that have not responded to either medical therapy or full- or partial-mouth extraction. There is currently no FDA-approved cell therapy for treating FCGS.
“FCGS is a terribly debilitating, immune-mediated disease and sadly, the outlook for cats that do not respond to available treatments is bleak,” says Dr. Chad B. Maki DVM, VetCell’s chief veterinary medical officer, in a recent press release. “Our vision is that DentaHeal will dramatically improve the quality of life for cats with FCGS by completely resolving or substantially improving their condition and provide them with long-term remission and relief.”
Because some cats with FCGS require lifelong pain management, antibiotics, and immune-suppressing drugs, this new treatment may help improve quality of life and, in some cases, save cats’ lives.
“This is a massive milestone for VetCell Therapeutics USA. Our dedicated and passionate team has worked tirelessly to advance to the clinical stage, and I believe the work being put into R&D and clinical trials will position VetCell as the catalyst for cell therapy becoming a mainstay of veterinary medicine,” said Tom C.K. Yuen, founder and chairman of PrimeGen Global, the parent company of VetCell Therapeutics.
This clinical trial, for which recruitment is now underway, will be conducted in collaboration with the UC Davis Veterinary Institute for Regenerative Cures. Click here to learn more about DentaHeal and the FCGS stem cell trial.