Fluid therapy and the role of the endothelial glycocalyx
Knowing how to approach critical patients is key.
Fluid therapy is a dynamic topic in the medical field. In the 19th century (and for many centuries prior), medical doctors thought bloodletting was a reasonable treatment for a variety of conditions. Fast-forward to the 1950s and beyond, when the mainstay of treatment became aggressive intravenous (IV) fluid boluses. Until recently, fluid boluses were routinely given to most patients in an emergency setting. However, recent studies in the human and veterinary fields have demonstrated that aggressive fluid therapy is not always beneficial and may often be detrimental to our patients. This article will review the fundamentals of fluid therapy, how to approach critical patients with regard to fluid therapy, and the consequential role of the endothelial glycocalyx.
Part 1: Fundamentals of fluid therapy
Calculating maintenance fluid requirements
Defining maintenance rate is often a point of contention. Some sources state the general rule of thumb for maintenance requirements should be 40 to 60 mL/kg/day. The problem with this estimation is that it is less accurate in very small and very large patients. Maintenance requirements are based on body surface area, which is a logarithmic measurement, not linear.
A more accurate measurement is based on metabolic rate; it is estimated that 1 kcal = 1 mL H2O. The simplest method to calculate basal metabolic rate or basal energy requirement (BER) is: [30 × BW(kg)] + 70. This has been shown to be accurate for patients over 2 kg and under 100 kg. In patients outside this range, a more accurate calculation is [BW (kg)^0.75] × 70. Estimating their maintenance based on BER alone will be slightly under their maintenance needs because BER assumes no activity. Consequently, using a multiplier between 1.2 and 2 may better approximate the true metabolic expenditure of the patient, and therefore their maintenance energy requirements.
However, given that fluid overload is more detrimental to a patient than being slightly dehydrated,1 the author’s preference is simply to use BER as the maintenance rate, especially because more fluid will be added to the total rate in order to account for replacing the estimated fluid deficit as well as ongoing losses. Any formula you come across for estimating maintenance fluid rates is just that—an estimate. This is why frequent monitoring of patients receiving IV fluids is so crucial.2
Routes of administration
Although IV fluid therapy is the most common route for hospitalized patients, there are multiple routes by which fluid therapy can be administered. Choosing which route depends on the patient, their specific needs, and their disease state.
Oral (per os)
Per os fluids are often forgotten and underrated. As long as there are no contraindications, such as vomiting or regurgitation, free access to water should always be allowed. In addition, fluids can be administered via a nasogastric tube if the patient is having one placed for other purposes.3 A classic example in which oral fluids can be very helpful in a critical setting would be a puppy with parvovirus enteritis (typically these patients are on CliniCare or another type of feeding with a high water content, but additional water boluses of 20-30 mL of lukewarm water can be given q2-4h.
Not only do enteral fluids stimulate the intestinal tract, but this is also the safest route of fluid administration in terms of avoiding fluid overload. In a patient with hypoalbuminemia, a heart murmur, or other comorbidities that may exacerbate hypervolemia, oral fluid administration should be considered. Oral fluids are contraindicated in any patient that has recently been vomiting or regurgitating.
Subcutaneous
Administering fluids subcutaneously is reasonable for rehydration; however, this is not an appropriate route of administration for patients exhibiting any signs of shock. Only isotonic solutions such as lactated Ringer’s solution (LRS) or Normosol-R (NSR) can be used, and they should be free of additives. The recommended dose is typically 30 to 40 mL/kg but can be higher in younger animals and exotic species. Subcutaneous fluids are contraindicated for any hypovolemic patient.
Intravenous
In any patient that is exhibiting signs of shock, not eating or drinking normally, anesthetized, or showing significant acid-base or electrolyte abnormalities, IV administration is the route of choice. IV access allows rapid volume resuscitation, correction of electrolyte abnormalities, and restoration of euglycemia in hypoglycemic patients. However, IV fluids are contraindicated in patients with congestive heart failure or any noncardiogenic causes of pulmonary edema. IV fluids should be used cautiously in patients with cavitary effusions, hypoalbuminemia, oliguric or anuric renal failure, heart murmur or gallop rhythm, or any other comorbidities that may contribute to fluid overload.
Intraosseous
In patients for which IV access cannot readily be obtained (ie, very small patients or some exotic species) and that are also too ill for subcutaneous fluid therapy, intraosseous fluids should be considered. After volume resuscitation using an intraosseous catheter, it is often possible to place an IV catheter, because improved perfusion will expand peripheral vessels.
Types of fluids
Crystalloids
Crystalloids are the mainstay of fluid therapy. Crystalloids consist of salt in water. Balanced crystalloid solutions also contain electrolytes and a buffer. Crystalloids rapidly redistribute out of the vascular space and into the interstitial space. It is estimated that 75% to 80% of the volume administered will move out of the vascular space within 30 to 60 minutes after administration. Larger volumes of crystalloids are required to have the same hemodynamic effect on a hypovolemic patient as a smaller volume of hypertonic saline (HSS) or a colloid solution. There are many crystalloid options available, but this article will focus on normal saline, balanced isotonic solutions, and HSS.
1. Normal saline
Normal saline (0.9% NaCl) used to be the mainstay of crystalloid fluid therapy in human medicine. However, studies have shown that normal saline may result in hypernatremia and hyperchloremia, as well as metabolic acidosis. It is important to note that normal saline is not a physiologic solution; it contains far more sodium and chloride than plasma and is acidifying by nature. Studies have also shown that hyperchloremia can lead to impaired renal function and an increased need for renal replacement therapy.4
In veterinary medicine, there are several circumstances in which normal saline can be useful. The first is in hypercalcemic patients, because it promotes calciuresis (sodium competes with calcium for tubular resorption). The second condition is in patients with hypochloremic metabolic alkalosis (which is rare but most commonly associated with an upper gastrointestinal obstruction). Furthermore, normal saline can be used to slowly correct severely hypernatremic patients with either a chronic condition or suspected chronic hypernatremia (correction of sodium abnormalities is complex and the intricacies of such are beyond the scope of this article). Normal saline should not be considered the first-choice crystalloid for IV fluid therapy, and it is the author’s belief that it should not be used outside of the 3 circumstances listed above.
2. Balanced isotonic solutions
Balanced crystalloid solutions are the most physiologic in nature, meaning their composition is most similar to that of plasma, in terms of content and tonicity (290-310 mOsm/L). They contain electrolytes as well as a buffer (lactate, acetate, or gluconate). The most commonly used isotonic crystalloids are NSR, LRS, and Plasma-Lyte (P-lyte). NSR and P-lyte have a sodium content most similar to that of plasma (both are 140 mEq/L), whereas LRS is slightly hypotonic (130 mEq/L with a tonicity of 273 mOsm/L).5 LRS is contraindicated in patients with severe liver diseasebecause hepatic function is necessary to convert lactate to bicarbonate. If liver function is impaired, the use of LRS may result in lactic acidosis.
3. Hypertonic saline
HSS differs from other crystalloids in that it is a good plasma expander. HSS typically comes in a solution of 5% to 7.5% sodium chloride. Hypertonicity causes water to shift from the interstitial and intracellular spaces into the intravascular space, which increases plasma volume. The volume of HSS required to produce specific hemodynamic effects is significantly lower than the volume of crystalloid required to produce the same effects, so HSS is ideal for low-volume fluid resuscitation.6 HSS should only be given to patients with normal interstitial and intracellular hydration. For example, HSS is useful for patients with acute hemorrhage or hypovolemic shock secondary to trauma; however, it would be a poor choice in a markedly dehydrated cat with renal failure. The recommended dosing is 3 to 5 mL/kg in dogs and 2 to 4 mL/kg in cats, usually given over 10 to 20 minutes. It is important to keep in mind that additional crystalloids should be given after the administration of HSS to avoid subsequent hypernatremia and interstitial dehydration.
HSS has also been used in patients with traumatic brain injury to reduce intracranial pressure. There have also been studies showing the beneficial effects of HSS on microvascular perfusion and oxygen delivery for patients in hemorrhagic shock. The literature is mixed in terms of the effect of HSS on coagulation; one study published in 2015 showed impaired platelet function and whole blood coagulation following HSS administration,7 whereas a study published in 2020 showed no effect on coagulation parameters.8 A single bolus given to stabilize an actively hemorrhaging patient, prior to the administration of blood products, is unlikely to have significant effects on coagulation, and should be considered in such patients for low-volume resuscitation.
Colloids
Colloids contain high molecular weight particles, which cannot cross an intact vascular barrier, and thereby provide significant oncotic support and volume expansion. Therefore, this should reduce the development of interstitial tissue edema, because the fluid remains in the intravascular space. Colloids can be further classified into natural or synthetic colloids.
1. Synthetic colloids
Hydroxyethyl starches (HES) are the most commonly used synthetic colloid in veterinary medicine. Dextrans and gelatins are the other 2 classes, but they are used less frequently. Hetastarch is the most commonly used HES in veterinary medicine. The benefits of using a synthetic colloid such as hetastarch include maintaining colloid osmotic pressure, low volume resuscitation, rapid intravascular expansion, and a volume of distribution that not only stays within the intravascular space but lasts much longer than it would with crystalloids (up to 6 hours). However, despite these advantages, there is no clinical evidence to support the use of synthetic colloids to reduce morbidity and mortality. In fact, most studies report increased mortality with the use of synthetic colloids, and the most significant adverse effects (AEs) include acute kidney injury and coagulopathies. Other less common but potentially severe AEs include allergic reactions, hepatopathy, pruritus, and reticuloendothelial dysfunction.9 In human medicine, synthetic colloids have fallen out of favor for the treatment of septic shock, as there have been multiple studies showing increased mortality with their use.
Synthetic colloids can still be considered but must be used with caution and should be avoided in any animal with impaired renal function, coagulopathy, hypervolemia, or heart murmur. The most recent Surviving Sepsis Campaign10 recommendations in human medicine advise against the use of synthetic colloids in patients with sepsis or septic shock; it is no longer common practice for veterinarians to use synthetic colloids in such patients. If used, the recommended dose range should not exceed 20 mL/kg/d to minimize or prevent any AEs. This can be given either as a continuous rate infusion or in incremental boluses of 5 mL/kg. In cats, the dose should not exceed 10 mL/kg/d and boluses can be given in increments of 2.5-3 mL/kg/d. Frequent monitoring of patients receiving synthetic colloids is imperative.
2. Natural colloids
In human medicine, 4% albumin is a mainstay of therapy in critically ill patients for a multitude of reasons beyond its colloidal benefit: it helps to maintain and restore the integrity of the endothelial glycocalyx, has anticoagulant properties, protects against oxidative damage, and plays a role in serum drug and hormone binding. Unfortunately, a readily available, species-specific albumin is difficult to obtain in veterinary medicine. Lyophilized canine-specific albumin is sporadically available, and in a small study of 7 dogs with septic peritonitis11 it showed improved outcomes. Given the lack of availability of canine-specific products, human serum albumin (HSA) has been used in veterinary species as well. However, some reports have shown severe and even fatal type III hypersensitivity reactions in dogs treated with HSA. Therefore, the use of HSA is controversial and not widely accepted at this time.
Blood component therapy such as fresh frozen plasma (FFP) can also be considered for colloidal support. Although FFP is typically used for patients with coagulopathies or acute hemorrhage, it also provides other components such as immunoglobulins and albumin, among other plasma components. Although FFP does contain albumin, it takes a significant volume of FFP to increase the concentration of albumin; it is estimated that 45 mL/kg will increase the albumin concentration by 1 mg/dL, and the normal dose for a coagulopathic patient is 10 to 20 mL/kg. Not only is this often cost prohibitive, but administering this volume increases the risk of transfusion-associated AEs. These include transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and febrile nonhemolytic transfusion reactions.
Part 2: Making a fluid therapy plan and monitoring patients on fluids
Fluids are drugs, and we should put just as much thought into fluid therapy for every patient as we do into administering any other medication. There are 4 main questions to ask yourself when formulating a fluid plan:
- Is the patient hypovolemic?
- Is the patient dehydrated?
- What is the estimated maintenance rate?
- What are the ongoing losses?
Treatment of hypovolemia is addressed in more detail in the next section. Once a patient is volume resuscitated, you can consider the latter 3 questions. If you have given fluid boluses, a repeat physical examination and blood work are helpful to reassess hydration status. For example, if the patient arrived with a packed cell volume/plasma total solids (PCV/TS) value of 65/9.0, then rechecking this parameter will help you know how much progress has been made in correcting the dehydration.
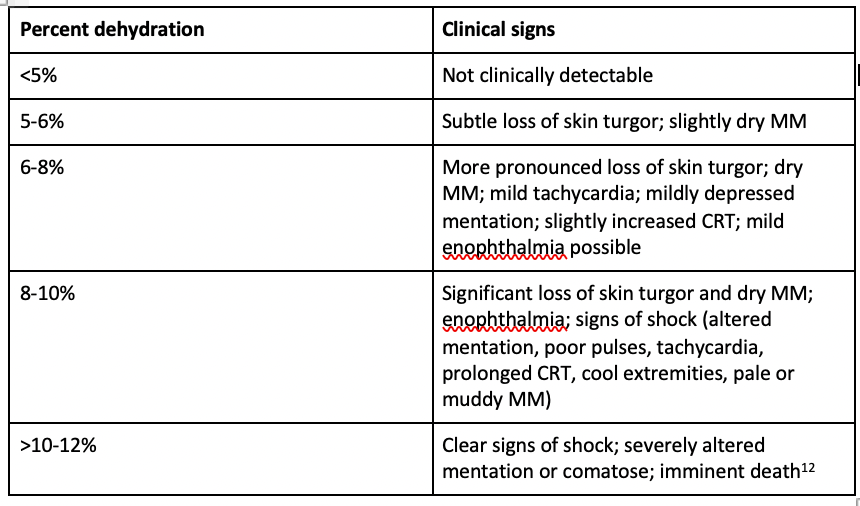
Many parameters can be used to determine the degree of dehydration (see Table below). The volume to be replaced can be calculated as [weight in kilograms × % dehydration] × 1000 = volume in liters to be replaced. Dehydration should be corrected over the first 12 to 24 hours; however, this should be extended to 36 to 48 hours in patients more susceptible to fluid overload (especially cats). Always keep in mind that if a problem happens slowly, it should be corrected slowly, and that dehydration and hypovolemia are not synonymous. Giving a fluid bolus for a patient who is dehydrated but not hypovolemic is contraindicated; it will not restore their interstitial deficit any faster and will damage the endothelial glycocalyx in the process.
Table.
Once you have calculated an estimated fluid deficit, estimate the patient’s maintenance needs as discussed above. Be sure to take into account any comorbidities, such as known cardiac disease, hypoalbuminemia, or any other factors that may predispose the patient to fluid overload. When in doubt, err on the side of caution and choose a lower rate (you can always give more fluid—it is much harder to take it back).
Finally, estimate the patient’s ongoing losses. If the patient has a urinary catheter in place, this can easily be used to quantify urine output every 4 to 6 hours. However, it is more difficult to estimate other kinds of losses, such as those from the intestinal or respiratory tract. In smaller patients, it may be helpful to weigh used potty pads and subtract the original weight of the pad (the difference in kg is equal to the volume lost in liters). One of the easiest ways to measure fluid balance is simply to weigh the patient every 12 hours.12 Other parameters that should be monitored include vital signs and mentation, blood pressure, PCV/TS, renal values, urine-specific gravity, blood gas and base deficit, serum lactate,2 and AFAST/TFAST (abdominal and thoracic focused assessment with sonography for trauma, triage, and tracking, especially if there are concerns about third spacing).
Once you have determined these parameters, you can calculate your fluid rate as follows:
fluid rate = (volume of dehydration in liters/number of hours to correct dehydration) + (maintenance rate) + (estimated ongoing losses). Once you have a final rate, be sure to ask yourself whether the fluid rate is appropriate for your patient’s weight. In general, it is not recommended to exceed rates of 2 to 3 mL/kg/h in cats or 2 to 6 mL/kg/h in dogs.
The most important part of making a fluid plan is to reassess. Fluid therapy is not one-size-fits-all. Pay attention to the parameters listed above and adjust as needed. For instance, if a patient comes in with a PCV/TS of 65/9.0 and a lactate level of 5mmol/L, but after 12 hours on fluids these numbers drop to 50/6.0 and 1.8 mmol/L, respectively, then that patient’s hydration status has significantly improved and the fluid rate should be adjusted accordingly. It is inevitable that potassium levels will decrease even in a healthy animal on fluids, but this is especially true in patients with disease states such as diabetic ketoacidosis or chronic kidney disease. Be sure to recheck electrolytes every 6 to 12 hours and add potassium as indicated.
Part 3: The approach to the critical patient and the role of the endothelial glycocalyx
Administering fluids to an overall healthy patient, such as one undergoing ovariohysterectomy or a dental procedure, differs tremendously from administering fluids to an ill patient. In addition to dealing with replacing volume deficits and treating shock, these patients have underlying conditions that often set off a systemic inflammatory cascade and predispose them to complications from fluid therapy. Restoring homeostasis in these patients is a challenge, and their specific needs can change by the hour or even the minute.
Approach to the critical patient
Rapid assessment of a critical patient’s perfusion parameters upon their arrival at the clinic is imperative to determine whether clinical signs of hypovolemia are present, what interventions are necessary, and to monitor the patient’s response to treatment. Hypovolemic patients will exhibit signs of hypoperfusion, including tachycardia (progressing to bradycardia in later stages of shock, and bradycardia is more common in cats), hypothermia, poor pulse quality, changes in mucous membrane color (pallor, gray/muddy), hypotension, and dull mentation. It is important to rule out cardiogenic shock as a cause of hypoperfusion, because fluid administration to patients in heart failure will worsen their disease state. If there is the presence of a heart murmur or gallop rhythm, dyspnea, abnormal lung sounds, or jugular distension, thoracic radiographs should be performed prior to making the decision as to whether fluid therapy is necessary. Any patient exhibiting signs of hypoperfusion should be provided flow-by oxygen immediately upon arrival.
If cardiogenic shock is ruled out, fluid resuscitation can be initiated while additional diagnostics are being performed. The shock volume in dogs is ~90 mL/kg, and in cats ~60 mL/kg.2 The recommendation is to administer a quarter of the shock volume (ie, approximately 20-25 mL/kg in dogs, 10-12 mL/kg in cats) over 20 minutes and reassess the patient’s perfusion parameters upon completion of the bolus.12 In most cases, more information will be available after 20 minutes on completion of point-of-care ultrasound and blood work. At this point, you can determine whether additional boluses or other treatments, such as blood products, are necessary.
If you have determined that a patient is neither interstitially dehydrated nor hypernatremic, but still exhibiting signs of hypovolemia, it is reasonable at this point to administer a dose of HSS. HSS can be especially helpful in patients showing signs of acute hemorrhage (ie, trauma, hemoabdomen), because volume-restricted resuscitation is advocated to prevent dislodgement of any blood clots and potentially worsened hemorrhage. In many cases, administering a single 20 mL/kg crystalloid bolus along with 4 to 5 mL/kg of HSS will help maintain a systolic blood pressure between 60 and 90 mmHg (permissive hypotension) until blood products can be administered and the patient can proceed to surgery. HSS should also be considered as part of a low-volume resuscitation protocol in patients with head trauma.
If a patient is determined to be septic, crystalloid boluses at 10 to 20 mL/kg can be administered while hemodynamic variables are being monitored. If half of the shock dose has been administered and there is still significant hypotension, early vasopressor therapy should be considered.13 These guidelines are based on multiple human studies, because there are no currently available guidelines regarding the use of IV fluids in animals with septic shock. Again, avoidance of the use of synthetic colloids in septic patients is important to prevent AEs associated with such products.
In the resuscitative phase of fluid therapy, frequent monitoring and reevaluation of perfusion parameters is important to determine the response to therapy and avoid fluid overload.3 In addition to the physical examination parameters listed above, other end points that can be used to evaluate perfusion more objectively include lactate, base deficit, and oxygen-transport variables. In human medicine, multiple studies have shown that reliance on traditional end points of resuscitation alone are insufficient in evaluating ongoing tissue hypoxia. Methods such as gastric tonometry, which specifically evaluate the perfusion of regional tissue beds, are also helpful in evaluating hypoxia, but many of these methods are not readily available in most veterinary settings. In critically ill patients, it is important to not only monitor traditional end points including blood pressure, heart rate, body temperature, capillary refill time, and urine output, but to also recheck values such as lactate, base deficit, blood gas analysis, and pulse oximetry with relative frequency, until these parameters begin to normalize.
Targeted end points of resuscitation in patients with shock include the following: systolic blood pressure, 80 to 100 mmHg; mean arterial pressure, 60 to 80 mmHg; base deficit –2 to 2 mEq/L; lactate 1 plus or minus 0.5 mmol/L; SvO2 greater than 70%; and pH greater than 7.32.14
What is the endothelial glycocalyx and why is it important?
The endothelial glycocalyx has garnered much attention in recent years, and for good reason. The endothelial glycocalyx is a gel-like matrix that lines all vascular endothelial surfaces. It is composed of glycosaminoglycans, proteoglycans, and glycoproteins. It is a dynamic structure that has many functions, including hemostasis, inhibition of microthrombosis, regulation of adhesion and migration of leukocytes through endothelial cells, regulation of vascular tone and permeability, and regulation of fluid flux across endothelial cells. Many plasma proteins interact with the endothelial glycocalyx, including albumin, antithrombin III, and superoxide dismutase.15
The results of damage to the endothelial glycocalyx can be catastrophic. Increased vascular permeability leads to capillary leakage and edema. Dysregulated cell-cell interactions lead to a proinflammatory and hypercoagulable state. Normal vascular reactivity and vessel tone are compromised. Shedding of the endothelial glycocalyx layer plays a prominent role in the pathophysiology of various critical illnesses, and care should be taken to reduce its damage.15
There are many potential causes of damage to the endothelial glycocalyx, including sepsis and systemic inflammatory response syndrome, hemorrhage, hyperglycemia, ischemia-reperfusion injury, and hypervolemia. In the context of this article, it is important to understand that a hypervolemic state (fluid overload) leads to endothelial glycocalyx disruption. In addition, rapid administration of crystalloids or colloids can increase endothelial glycocalyx shedding.15 Therefore, it is important to always determine whether a fluid bolus is required in the first place, and why it is important to always be reevaluating a patient’s response to fluid therapy.
Many investigative studies have explored possible therapies to preserve or repair the endothelial glycocalyx. At this time, there does not appear to be one particular therapy that is strongly recommended, nor is there any available research specific to veterinary medicine. Treatments that have shown promise include early blood product administration, matrix metalloproteinase inhibitors, albumin, exogenous glycosaminoglycans, activated protein C, sulodexide, and heparin.
Because little is known about these treatments and many of them are not readily available, our best option as veterinary professionals is to make every effort to avoid further damage to the endothelial glycocalyx. A judicious, calculated approach to fluid therapy should be taken with every patient.
References
- Cavanagh AA, Sullivan LA, and Hansen BD. Retrospective evaluation of fluid overload and relationship to outcome in critically ill dogs. J Vet Emerg Crit Care (San Antonio). 2016;26(4):578-586. doi:10.1111/vec.12477
- 2013 AAHA/AAFP Fluid Therapy Guidelines for Dogs and Cats. ImplementationToolkit. American Animal Hospital Association. 2013. Accessed July 12, 2021. https://Www.Aaha.Org/Globalassets/02-Guidelines/Fluid-Therapy/Fluidtherapy_Guidlines_Toolkit.Pdf
- Londoño L. Fluid Therapy in Critical Care. Today’s Veterinary Practice. Accessed July 11, 2021. https://todaysveterinarypractice.com/fluid-therapy-in-critical-care/
- Kim HY, Nam A, Song KH, Youn HY, Seo KW. Effect of 7.5% hypertonic saline solution on whole blood coagulation in healthy dogs using thromboelastography. J Vet Emerg Crit Care (San Antonio). 2020;30(4):442-448. doi:10.1111/vec.12959
- DiBartola SP, Bateman S. Introduction to fluid therapy. In: DiBartola SP, ed. Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice. Elsevier-Saunders; 2012:339.
- Muir WW. Crystalloids or colloids? dvm360.com.! February 1, 2013. Accessed July 12, 2021. https://www.dvm360.com/view/crystalloids-or-colloids
- Wurlod VA, Howard J, Francey T, Schweighauser A, Adamik KN. Comparison of the in vitro effects of saline, hypertonic hydroxyethyl starch, hypertonic saline, and two forms of hydroxyethyl starch on whole blood coagulation and platelet function in dogs. J Vet Emerg Crit Care (San Antonio). 2015;25(4):474-487. doi:10.1111/vec.12320
- Kundra P, Goswami S. Endothelial glycocalyx: Role in body fluid homeostasis and fluid management. Indian J Anaesth. 2019;63(1):6-14. doi:10.4103/ija.IJA_751_18
- Cazzolli D, Prittie J. The crystalloid‐colloid debate: consequences of resuscitation fluid selection in veterinary critical care. J Vet Emerg Crit Care (San Antonio). 2015;25(1):6-19. doi:10.1111/vec.12281
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486-552. doi:10.1097/CCM.0000000000002255
- Craft EM, Powell LL. The use of canine-specific albumin in dogs with septic peritonitis. J Vet Emerg Crit Care (San Antonio). 2012;22(6):631-639. doi:10.1111/j.1476-4431.2012.00819.x
- Lee JA, Powell L. Fluid therapy in real-life practice: all you need to know!June 25, 2014. Accessed May 7, 2020. https://vetgirlontherun.com/wp-content/uploads/2014/06/FluidTherVG625141.pdf
- Lat, I., Coopersmith, C.M., De Backer, D. et al. The surviving sepsis campaign: fluid resuscitation and vasopressor therapy research priorities in adult patients. ICMx 9, 10 (2021). https://doi.org/10.1186/s40635-021-00369-9
- Prittie J. Optimal endpoints of resuscitation and early goal‐directed therapy. J Vet Emerg Crit Care. 2006;16(4):329-339. doi:10.1111/j.1476-4431.2006.00160.x
- Gaudette S, Hughes D, Boller M. The endothelial glycocalyx: structure and function in health and critical illness. J Vet Emerg Crit Care (San Antonio). 2020;30(2):117-134. doi:10.1111/vec.12925