Ophthalmology Challenge: Aggressive ulcerative keratitis in a dog
A 4-year-old, 66-lb (30-kg), spayed female greyhound was presented to the Ophthalmology Service at the University of Florida's College of Veterinary Medicine for ophthalmic evaluation.
A 4-year-old, 66-lb (30-kg), spayed female greyhound was presented to the Ophthalmology Service at the University of Florida's College of Veterinary Medicine for ophthalmic evaluation. Two days before referral, the referring veterinarian had identified a corneal ulcer in the dog's left eye based on clinical appearance and the result of fluorescein staining.
The referring veterinarian had initiated topical treatment of the left eye that included administering 0.3% gentamicin sulfate ophthalmic solution four times a day, 5% acetylcysteine solution every two hours, autogenous canine serum four times a day, and 1% atropine sulfate solution once a day. After two days of this treatment, the corneal ulcer had progressed in diameter and depth, and the dog showed signs of increased pain, so the veterinarian referred the case. The dog had no history of trauma to the eye and no other health problems and was receiving no other medication.
Physical and ophthalmic examination findings
The dog was mildly depressed on physical examination. The results of the remainder of the physical examination were normal except for the left eye, which exhibited profound blepharospasm with severe conjunctival hyperemia and moderate epiphora. Both pupils were mobile and midrange in examination room light. Schirmer tear test results were 10 mm/min in the right eye and 18 mm/min in the left eye (normal ≥ 15 mm/min). The menace response, dazzle reflex, and pupillary light reflexes (direct and consensual) were present and bilaterally normal. The corneal lesion in the left eye stained with fluorescein (Figure 1). It was a round central corneal ulcer that was 8 mm in diameter and greater than half the stromal depth; the ulcer had a deeper pit in the center. Corneal melting and slight corneal neovascularization were present.
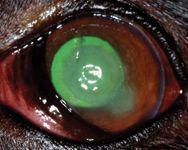
Figure 1. The dog's left eye stained with fluorescein on presentation (Day 1).
Slit-lamp biomicroscopy revealed a moderate amount of corneal edema and mild aqueous flare in the left eye. Intraocular pressures were not measured because of the dog's stressed condition. No apparent abnormalities were noted in the lens, vitreous, or fundus of either eye. We obtained corneal swab samples from the left eye for aerobic bacterial and fungal culture. Samples for corneal cytologic examination were not obtained because of the patient's fractious nature.
Differential diagnoses
Our initial diagnosis based on the dog's history and ophthalmic examination and fluorescein staining findings was a melting, stromal corneal ulcer. Clinical signs consistent with ulcerative keratitis included miosis, blepharospasm, epiphora, and photophobia. Causes of corneal ulceration in dogs include trauma, keratoconjunctivitis sicca, exposure keratitis, a foreign body, distichiasis, entropion, trichiasis, ectopic cilia, and exposure to caustic substances. No initiating factors were identified on initial ophthalmic examination in this patient. Given the gelatinous clinical appearance of the left cornea in this case, the likely differential diagnoses included corneal ulceration with stromal proteolysis due to secondary bacterial or mycotic infection and sterile melting corneal ulceration due to exposure to a caustic substance. Confirmation of infectious keratitis is based on the results of culture, corneal cytology, and histopathology and, presumptively, on response to treatment. We thought the subnormal Schirmer tear test result in the right eye was due to early or mild keratoconjunctivitis sicca or atropine administration.
Initial treatment
We initiated medical management to suppress the proteolytic activity and sterilize the ulcer. The owner elected to treat the dog on an outpatient basis. We discontinued the gentamicin ophthalmic solution prescribed by the referring veterinarian and initiated tobramycin ophthalmic solution given every four hours, and we increased the autogenous canine serum administration frequency to six times a day. The acetylcysteine and atropine were continued. We selected tobramycin because it causes less inhibition of corneal epithelial migration as well as fewer cytopathologic effects than gentamicin does.1
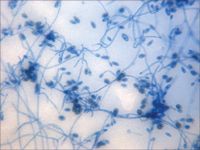
Figure 2A. A photomicrograph of a tape preparation of the fungal culture from the left eye on Day 3. Septate, hyaline, branching hyphæ with ellipsoidal conidia are present (lactol phenol cotton blue stain, 100Ã).
No marked change was apparent in the eye the next day (Day 2). Superficial vessels had begun to migrate inward from the dorsal limbus by Day 3. No aqueous flare was present in the left eye on Day 3. Because the eye continued to appear painful and the pupil was not fully dilated, we added carprofen (2.5 mg/kg orally b.i.d.) and increased the topical atropine administration frequency to twice a day.

Figure 2B. Cytologic examination of a corneal scraping of the left eye on Day 3 showed oval-shaped, palely basophilic, sporelike structures (arrows) amid the basophilic cellular debris and corneal epithelial cells (Wright's-Giemsa stain, 100Ã).
Diagnostic tests
On Day 3, results from the corneal fungal cultures were positive for scant growth of Acremonium species (Figure 2A); there was no growth on aerobic bacterial culture. The cornea was anesthetized with topical 0.5% proparacaine hydrochloride, and samples were collected from the left cornea with the handle end of a scalpel blade for cytologic examination. The samples stained with Wright's-Giemsa revealed basophilic cellular debris, along with a moderate number of corneal epithelial cells and nondegenerative neutrophils and several sporelike structures consistent with fungal infection (Figure 2B). Budding yeast structures and hyphal fragments were observed with Gomori's methenamine silver stain (Figure 2C).
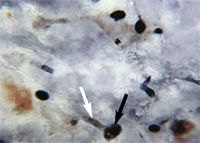
Figure 2C. Cytologic examination of a corneal scraping of the left eye on Day 3 also revealed dark-brown-staining budding yeast (black arrow) and hyphal fragments (white arrow) (Gomori's methenamine silver stain, 100Ã).
Diagnosis and additional treatment
We diagnosed keratomycosis based on the clinical presentation and the fungal culture and corneal cytology results. Unfortunately, because of this dog's fractious nature, we could not perform corneal cytology at the initial presentation, so specific antifungal therapy was delayed until culture results were available. We modified the therapy on Day 3 by initiating 5% natamycin suspension (Natacyn—Alcon Laboratories) given in the left eye three times a day and decreasing the tobramycin administration to three times a day.
On Day 5, the ulcerated corneal stroma appeared firmer and less gelatinous, the pupil was 75% dilated, the limbal vessels continued to migrate toward the lesion, and the blepharospasm and conjunctival hyperemia had improved slightly. A brown-pigmented ring had formed around the edge of the corneal opacity (Figure 3). We increased the natamycin administration frequency to every four to six hours in the left eye, and we discontinued the acetylcysteine. Schirmer tear test results in the left eye had decreased to 8 mm/min.
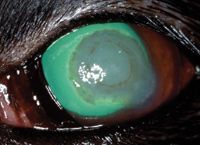
Figure 3. The left eye on Day 5 stained with fluorescein stain. Note the brown-pigmented ring around the center of the corneal opacity and limbal vessels migrating toward the lesion.
On Day 9, Schirmer tear test results had increased to 16 mm/min in the left eye and 14 mm/min in the right eye. A brown corneal lesion had formed in the center of the corneal opacity of the left eye; the lesion had a rough center and a smooth peripheral ring. The dog appeared to be more comfortable, and the peripheral cornea was smoother. Both superficial and deep corneal vessels continued to migrate toward the lesion. We decreased the atropine administration frequency to once a day and continued the remaining treatments in the left eye (natamycin every four hours, autogenous canine serum q.i.d., tobramycin t.i.d.). We also continued the oral carprofen.
On Day 12, the fluorescein-positive area had decreased to 9 x 6 mm, and the brown ring had become more prominent. Schirmer tear test values were 9 mm/min in the right eye and 15 mm/min in the left eye. Peripheral corneal neovascularization had extended 3 mm from the limbus. A repeat corneal culture produced scant growth of Acremonium species. Therapy was unchanged except for adding artificial tears in the right eye twice a day.
On Day 15, the left eye appeared less painful and had less discharge. Corneal vessels had reached the edge of the lesion. Clearing in the periphery was present, and the brown-pigmented ring was gone (Figure 4); however, the plaque was still present. The fluorescein-positive area measured 9 x 5 mm. Therapy was continued unchanged.
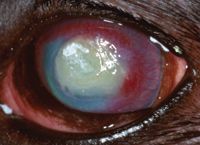
Figure 4. The left eye on Day 15 stained with fluorescein. Corneal vessels have reached the lesion's edge. Clearing in the periphery is present, and the brown-pigmented ring is no longer apparent.
On Day 19, the owner reported that the dog exhibited fewer signs of pain and held the eye open more frequently. The iris was now clearly visible. The fluorescein-positive area measured 8 x 6 mm. Corneal vessels remained at the edge of the stained region, with the limbal cornea continuing to clear (Figure 5). No changes were made in the treatment plan.
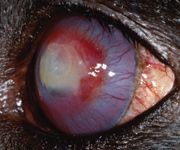
Figure 5. The left eye on Day 19 stained with fluorescein. The fluorescein-positive area measures 8 x 6 mm. Corneal vessels remain at the lesion's edge, with the limbal cornea continuing to clear.
On Day 26, Schirmer tear test values were 14 mm/min in the right eye and 12 mm/min in the left eye. The owner reported that the dog was squinting occasionally. Applanation tonometry showed an intraocular pressure of 13 mm Hg in both eyes. The left pupil was widely dilated. The fluorescein-positive area had decreased markedly, measuring only 6 x 3 mm. Corneal vessels had progressed to the edge of the plaque, and the corneal stroma was continuing to clear peripherally (Figure 6). We modified the therapy by decreasing carprofen administration to once a day and discontinuing the artificial tears in the right eye.
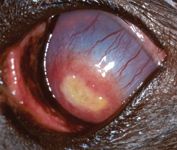
Figure 6. The left eye on Day 26 stained with fluorescein. The fluorescein-positive area has decreased markedly, measuring only 6 x 3 mm with corneal vessels perfused to the lesion's edge. The corneal stroma is continuing to clear peripherally.
On Day 33, the owner reported that the dog's left eye appeared comfortable and was always wide open. The entire cornea was fluorescein-negative. We decreased both the natamycin and autogenous canine serum frequencies to three times a day, decreased atropine administration to every other day, continued tobramycin at three times a day, and discontinued the oral carprofen.
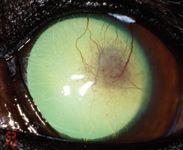
Figure 7. The left eye on Day 43 stained with fluorescein. The cornea is fluorescein-negative with only ghost vessels and granulation tissue remaining.
On Day 43, the left cornea remained fluorescein-negative. The dog showed no discomfort in the left eye, and only ghost vessels and granulation tissue remained (Figure 7). We discontinued all medication at this point. A final visit on Day 82 revealed only a white stromal scar with few perfused vessels and no granulation tissue (Figure 8).
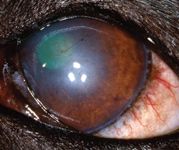
Figure 8. The left eye on Day 82. A white stromal scar remains with few perfused vessels and no granulation tissue.
Discussion
Melting corneal ulcers result from excessive production of proteolytic enzymes that digest the corneal stroma. The clinical appearance of melting corneal ulcers includes a gelatinous and liquefied corneal stroma with corneal edema. Ocular pain, mucopurulent ocular discharge, and secondary uveitis often accompany the process. Proteases are normally produced by the corneal epithelium, keratocytes, and neutrophils to aid in corneal repair.2 Certain bacteria and fungi, most notably Pseudomonas species, can also produce these enzymes, resulting in rapid digestion of the corneal stroma and possibly leading to corneal perforation.2 Melting corneal ulcers may indicate an infectious etiology, but nonseptic insults such as exposure to certain caustic agents, especially alkaline substances, may activate the matrix metalloproteinase.
Fungal keratitis is infrequently reported in dogs but should be considered as a differential diagnosis for keratitis. The typical appearance of keratomycosis in any species is a corneal ulcer, a plaque with white fluffy stromal infiltrates, or both. Occasionally, dematiaceous fungi will produce brown corneal pigment.3 Fungi are opportunistic saprophytes that require damage to the corneal epithelium or decreased local immunity to establish an infection in the cornea.4 Most cases of keratomycosis in small animals are associated with prior corneal disease or trauma, systemic diseases such as hyperadrenocorticism or diabetes mellitus, or therapy with antibiotics or corticosteroids, any of which may predispose an animal to fungal infection.3,5-7 This patient had Schirmer tear test values below normal in both eyes during treatment, which could have been a predisposing factor in this case.8
Fungal keratitis in dogs generally presents as acute, progressive ulcers; keratitis characterized by plaque formation; or chronic, nonprogressive, superficial ulcers or erosions.3 The most commonly implicated fungal pathogen in dogs is Aspergillus species.3,9 Keratomycosis in canine corneas in previous reports was also caused by Fusarium,3 Acremonium,5 Curvularia,6 Pseudallescheria,7 and Alternaria species.10 Fungi were isolated from the conjunctiva in 11 of 50 (22%) normal dogs in one study.11 In a previous report in dogs with keratoconjunctivitis sicca, fungi were isolated from the corneal surface in nine of 61 eyes (15%), with Alternaria species being the most common (two of 61, 3%).10
Fungal infection is diagnosed based on both cytologic examination and culture performed on deep corneal scrapings.11,12 Cytologic examination is ideally performed by using a modified Gomori's methenamine silver stain of a corneal scraping, but hyphae can usually be seen with Wright's-Giemsa stain. Gomori's methenamine silver stain is reported to be 86% sensitive for identifying fungal elements in tissue.13 On cytologic examination, Acremonium species appear as septate, hyaline, branching hyphae 2- to 4-μm wide with tapering phialides.4 Fungal culture is most commonly performed by using Sabouraud's dextrose agar between 25 to 30 C; growth is generally evident within five days.4,14 Ideally, fungal sensitivity testing should be performed in cases of fungal keratitis, but results are usually not available for several weeks, thus diminishing the clinical utility of this diagnostic test.
Both corneal cytology and culture identified Acremonium species in this patient. Acremonium species are saprophytic fungi commonly found in decaying plant material and as soil contaminants; they infrequently infect people and dogs.5,14-16 Most commonly, Acremonium species infections in people lead to the formation of mycetomas or keratitis, although endophthalmitis and systemic infections can occur.14,16 Acremonium species infections have been previously reported in dogs, causing systemic infection and keratoconjunctivitis.5,15
Medical management of ulcerative fungal keratitis should include antimicrobials, nonsteroidal anti-inflammatory drugs (NSAIDs), collagenase or protease inhibitors, and parasympatholytics. The antifungal of choice for treating ocular Acremonium species infection in dogs is unknown. In people, topical antifungal therapy (natamycin 5% or amphotericin B 0.15%) and keratoplasty appear to effectively treat Acremonium species keratitis.14 Amphotericin B has the best in vitro activity against various Acremonium species.16 In one previous report of Acremonium species keratitis in dogs, treatment with natamycin and 2% yellow mercury oxide was effective.5 Members of this genus are also reported to be susceptible to posaconazole (Noxafil—Schering-Plough),17 but in this case a good response to natamycin was seen. Topical antifungal therapy should be applied three or four times a day initially, theoretically, to reduce iridocyclitis due to death of fungi, and then should be increased up to every four hours after the first few days.
Adjunctive therapy with broad-spectrum topical antibiotic therapy (neomycin-polymyxin B-gramicidin, gentamicin, chloramphenicol, or tobramycin)2,18,19 should be used with fungal keratitis to treat or prevent secondary bacterial keratitis. Topical or systemic NSAIDs should be used if anterior uveitis is present (aqueous flare, miosis, hypotonia). Topical collagenase and protease inhibitors are valuable in slowing the progression of melting corneal ulcers.18 The most commonly used protease inhibitors in veterinary medicine are acetylcysteine and autogenous serum applied four to six times a day. A topical parasympatholytic, 1% atropine sulfate solution, should be included in the treatment of ulcerative keratitis for its mydriatic and cycloplegic effects and to reduce synechiae formation. Superficial keratectomy of the fungal plaque can be performed with topical anesthesia to reduce the infectious burden and enhance penetration of topical medications. Surgery, consisting of either creating conjunctival flaps or grafts or performing corneal transplantation, should be considered in severe cases in which a descemetocele or a melting ulcer affecting greater than half the depth of the cornea is present.
Cases of canine fungal keratitis generally respond favorably to medical treatment, although corneal surgery or enucleation or both may be required in severe cases.3 In this case, only the decreased tear production was identified as a potential predisposing factor to explain the fungal keratitis. After about one month of topical antifungal treatment, the ulcer in this case healed, leaving only ghost vessels and granulation tissue in the cornea. Less than three months after initial presentation, the dog exhibited no pain and could see from its left eye, and only a faint white corneal scar remained.
The photographs and information for "Ophthalmology Challenge" were provided by Matthew P. Landry, DVM; Mary E. Lassaline, DVM, PhD; Andras M. Komaromy, DVM, PhD, DACVO; Maria E. Kallberg, DVM, PhD; Dennis E. Brooks, DVM, PhD, DACVO; and Kirk N. Gelatt, VMD, DACVO, Department of Small Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL 32610-0126.
REFERENCES
1. Hendrix, D.V. et al.: Effect of antibiotics on morphologic characteristics and migration of canine corneal epithelial cells in tissue culture. AJVR 62 (10):1664-1669; 2001.
2. Whitley, R.D.; Gilger, B.C.: Diseases of the canine cornea and sclera. Veterinary Ophthalmology, 3rd Ed. (K.N. Gelatt, ed.). Lippincott Williams & Wilkins, New York, N.Y., 1999; pp 635-673.
3. Marlar, A.B. et al.: Canine keratomycosis: A report of eight cases and a literature review. JAAHA 30:331-340; 1994.
4. Thomas, P.A.: Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 16 (4):730-797; 2003.
5. Mendoza, L. et al.: Canine mycotic keratoconjunctivitis caused by Acremonium kiliense. Sabouraudia 23 (6):447-450; 1985.
6. Qualls, C.W. Jr. et al.: Mycotic keratitis in a dog: Concurrent Aspergillus sp. and Curvularia sp. infections. JAVMA 186 (9):975-976; 1985.
7. Smedes, S.L. et al.: Pseudallescheria boydii keratomycosis in a dog. JAVMA 200 (2):199-202; 1992.
8. Moore, C.P.: Diseases and surgery of the lacrimal secretory system. Veterinary Ophthalmology, 3rd Ed. (K.N. Gelatt, ed.). Lippincott Williams & Wilkins, New York, N.Y., 1999; pp 583-607.
9. Gaskin, J.M.: Microbiology of the canine and feline eye. Vet. Clin. North Am. (Small Anim. Pract.) 10 (2):303-316; 1980.
10. Salisbury, M.A. et al.: Microorganisms isolated from the corneal surface before and during topical cyclosporine treatment in dogs with keratoconjunctivitis sicca. AJVR 56 (7):880-884; 1995.
11. Samuelson, D.A. et al.: Conjunctival fungal flora in horses, cattle, dogs, and cats. JAVMA 184 (10):1240-1242; 1984.
12. Massa, K.L. et al.: Usefulness of aerobic microbial culture and cytologic evaluation of corneal specimens in the diagnosis of infectious ulcerative keratitis in animals. JAVMA 215 (11):1671-1674; 1999.
13. Liesegang, T.J.; Forester, R.K.: Spectrum of microbial keratitis in South Florida. Am. J. Ophthalmol. 90 (1):38-47; 1980.
14. Fincher, R.E. et al.: Infection due to fungus Acremonium (Cephalosporium). Medicine 70 (6):398-410; 1991.
15. Simpson, K.W. et al.: Systemic mycosis caused by Acremonium sp. in a dog. JAVMA 203 (9):1296-1299; 1993.
16. Guarro, J. et al.: Acremonium species: New emerging fungal opportunists—In vitro antifungal susceptibilities and review. Clin. Infect. Dis. 25 (5):1222-1229; 1997.
17. Grooters, A.M.; Taboada, J.: Update on antifungal therapy. Vet. Clin. North Am. (Small Anim Pract.) 33:749-758; 2003.
18. Severin, G.A.: Severin's Veterinary Ophthalmology Notes, 3rd Ed. (G.A. Severin, ed.). Colorado State University, Fort Collins, Colo., 1995; pp 285-350.
19. Andrews, S.E.: Corneal fungal disease in small animals. Clin. Tech. Small Anim. Pract. 18:186-192; 2003.