Therapeutic caveats: Difficult urinary tract infections
Acute onsets of uncomplicated bacterial UTIs often "respond" to empirical antimicrobials.
As discussed in last month's Diagnote, the underlying causes of bacterial urinary tract infections (UTIs) encompass transient or persistent abnormalities in host defenses.
It follows that protocols designed to safely and effectively eliminate UTIs should include:
- Selection of appropriate antimicrobial agents to eradicate microbial pathogens, and
- Detection and treatment of host defense abnormalities that allow bacteria to colonize and invade the urinary tract.
The objective of the second part of this series is to summarize caveats derived from our experience with initial treatment of bacterial UTIs. Caveats associated with treatment of recurrent bacterial UTIs will follow in next month's Diagnote.
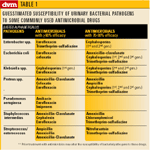
Table 1
Empirical use
Should empirical choice of antimicrobial drugs be considered to treat bacterial UTIs?
In context of bacterial UTI, empirical treatment consists of selection of an antibacterial drug without knowledge of the susceptibility of bacteria to that drug. It is based on: 1) previous experience with the types of bacterial pathogens most likely to cause UTI, 2) epidemiologic data about the susceptibility of these bacteria to various antimicrobial agents (Table 1), and 3) knowledge that the drug will attain an appropriate concentration in urine (Table 2, p. 6S).
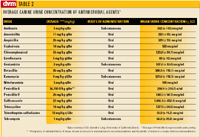
Table 2
The most commonly reported bacterial isolates from dogs with UTIs have been Escherichia coli (~45 percent), Staphylococcus spp. (~10 percent), Proteus spp. (~10 percent), Klebsiella spp. (~10 percent), Enterococcus spp. (~5 percent) and Streptococcus (~5 percent).
Caveat: Patients with acute onsets of uncomplicated bacterial UTIs often "respond" to empirical antimicrobial treatment. Therefore, making an empirical, but educated, "guess" of the proper antibiotic to treat patients with acute onsets of uncomplicated bacterial UTI has been an accepted standard of practice.
However, the standard of practice also includes appropriate follow-up evaluation of the patient to determine the efficacy of therapy. Empirical selection of antimicrobial agents is not recommended for patients that have 1) a history of frequently recurrent clinical signs, and 2) been given antibacterial drugs to treat signs of urinary tract disease in the past three to six weeks.
Susceptibility testing
When should antimicrobial agents be selected on the basis of antimicrobial susceptibility tests?
Evaluation of the susceptibility of infecting bacteria to antimicrobial drugs is advisable as the most reliable guide for choice of therapeutic agents because different bacterial pathogens isolated from dogs and cats with UTI may vary widely in their susceptibility to specific antimicrobial agents. Especially Pseudomonas aeruginosa and Klebsiella spp., but also Enterobacter spp., Escherichia coli and Proteus spp. are examples of urinary pathogens that may be associated with polyresistant strains. There are some circumstances when antimicrobial susceptibility tests are essential (Table 3).

Table 3
Caveat: Once bacterial urinary tract pathogens that have been exposed to one or more antimicrobial drugs, they often acquire resistance to many commonly used antimicrobial agents. In this setting, the susceptibility of bacterial pathogens is unpredictable, and therefore cannot be accurately predicted from tables derived from untreated patients (Table 1). If a patient with recurrent signs of UTI has recently been given antimicrobics, it is essential that the therapy be re-evaluated on the basis of quantitative culture and antimicrobic susceptibility tests to determine whether changes in susceptibility have occurred.
Difficult UTIs
What type of susceptibility test should be considered for difficult UTIs?
Two main types of antimicrobial susceptibility tests are currently in common use: 1) the disc diffusion susceptibility test, and 2) the antibiotic dilution susceptibility test. The agar disc diffusion test (Kirby-Bauer test) of antimicrobic susceptibility was primarily designed for treatment of infections based on knowledge of serum concentrations of the antimicrobial drug. Therefore, the concentration of antimicrobics (except nitrofurantoin) in the paper discs is comparable to expected serum concentrations of drugs when given at commonly used dosages. However, many drugs attain concentrations in urine that are 10 to 100 times greater than that observed in serum. Therefore, disc-diffusion tests underestimate the potential efficacy of many antimicrobial drugs in patients with lower urinary tract infections. Examples of some drugs that are excreted in high concentration in urine include ampicillin, cephalexin, chloramphenicol, enrofloxacin, oxytetracycline, penicillin G and trimethoprim). Agar diffusion antimicrobial susceptibility tests may be useful for patients with renal infections and acute bacterial prostatitis since renal and prostate tissue concentrations of antibiotics are more likely to correspond to serum concentrations of antibiotics.
The second type of commonly used susceptibility test is the antibiotic dilution susceptibility test. It is designed to determine the minimum concentration of the antimicrobial drug that will inhibit the growth of uropathogens (MIC). The MIC is then compared to antimicrobial concentrations expected to be present in urine following administration of therapeutic dosages of the drug. In general, the antimicrobial agent is likely to be effective in vivo if it can achieve a concentration of four times the MIC (Table 2, p. 6S). Note that the MIC is several dilutions lower than the minimum bactericidal concentration (MBC) of drugs.
Caveat: The MIC of an antimicrobial drug varies with each type of infecting pathogen. In addition, the MIC for the same type of pathogen may vary with different episodes of relapsing infections. A reliable way to assess the in vivo effectiveness of an antimicrobic in a timely fashion is to re-culture the urine during and after therapy.
Serum and urine concentrations
Why should serum and urine concentrations of antimicrobics be considered to treat difficult UTIs?
Serum or urine concentrations of antimicrobial agents do not necessarily reflect tissue concentrations. In context of this limitation, we make the following recommendations: 1) select agents that have high urine concentrations (at least four times the MIC) for treatment of lower urinary tract infections, and 2) select antimicrobial agents that attain high concentrations in serum and (if possible) urine for treatment of acute bacterial infections of the prostate gland or bacterial infections of the kidneys.
Caveat: Special precautions must be taken when selecting antimicrobial agents for treatment of patients with renal failure. If the standard dosage regimen is given to patients with reduced renal function, increased serum concentrations of drugs dependent on the kidneys for elimination from the body could predispose the patient to adverse drug reactions. Likewise, renal failure could reduce the effectiveness of drugs normally excreted in high concentrations in urine.
Dosages and maintenance schedules
What drug dosage and maintenance schedule should be selected?
To help eradicate bacterial pathogens from the site(s) of infection, the concentration of the drug must be maintained at varying concentrations above the MIC for at least a portion of the dosing interval. However, peak drug concentrations and the time drug concentrations remain above the MIC during the dosing interval varies with different antimicrobial agents and different sites of infection. In addition, recall that the MIC varies with different bacterial pathogens, and may vary with different episodes of relapsing infections caused by the same pathogen. Thus, the optimum dosage of an antimicrobial drug to treat bacterial UTI varies with the susceptibility of each pathogen, the sites of infection, and the integrity of host defense and repair mechanisms. These principles justify the value of flexible label dosing of various antimicrobial drugs such as enrofloxacin.
In general, dosage and intervals between maintenance dosages should conform to the recommendations of the manufacturer. Strive for maintenance of high concentrations of antimicrobial agents in urine to treat infections of the lower urinary tract, and high serum concentrations for treatment of infections of the renal parenchyma.
Frequency of administration of a drug is based on whether its antimicrobial activity is best correlated with, 1) the drug's peak serum concentration (concentration dependent), or 2) the time during the dosing interval that serum concentration of the drug is higher than the MIC (time dependent). For example, to maximize the value of drugs whose antimicrobial efficacy is time dependent (such as beta lactams), they should be given more frequently and not just at higher doses. The antimicrobial activity of fluoroquinolones is concentration dependent. The ability of companion animal owners to comply with recommendations must also be considered when selecting drug dosage intervals.
The volume of urine produced by the patient should be considered when selecting dosage for antimicrobial drugs. Higher dosages are needed to maintain effective urine concentrations of drugs in polyuric patients as compared to those who are producing concentrated urine. Although signs of UTI may subside following administration of suboptimum doses of an antimicrobial agent to polyuric patients, bacteriuria may persist.
To ensure adequate concentrations of the drug in the urinary tract during treatment intervals, it is recommended that daily doses be administered shortly following micturition, and especially just prior to a period of confinement during which voiding is not permitted (such as overnight).
Caveat: Ultimately, it is the responsibility of the clinician caring for the patient to balance issues of drug safety and efficacy in order to modify drug dosages and maintenance intervals according to individual needs.
Duration of treatment
How long should bacterial UTIs be treated?
Antimicrobic therapy should be continued for a sufficient period to: 1) eliminate bacteria from tissue in addition to urine, and 2) allow the urinary tract and its defense mechanisms time to recover sufficient function to prevent recurrence of UTI. Therefore, it is not possible to establish rigid timelines about the duration of antimicrobic therapy. Rather, the duration of therapy is dependent on each patient's response to therapy, and must be individualized on the basis of serial clinical and laboratory findings. Remission of clinical signs is not itself a reliable index of successful eradication of infection, nor is reduction in WBC, RBC and protein detected by urinalysis. Duration of therapy should be based on the persistent elimination of bacteria as defined by urine cultures in addition to amelioration of pyuria and clinical signs.
For the purpose of initial therapeutic plans, we recommend that the first episode of urinary tract infection in females and neutered males be treated for approximately 10 days to two weeks provided bacteria are not isolated from urine during therapy (Table 4). For intact male dogs at risk for bacterial prostatitis, three to four weeks of treatment is recommended. In general, continue therapy for one week after resolution of pyuria, hematuria and proteinuria as documented by urinalysis. If, after a suitable period of treatment with an antimicrobial agent, urine cultures are negative but urinalyses remain abnormal, an underlying abnormality is likely.

Table 4
For infrequently recurrent UTIs due to reinfections, treatment should be continued for two to three weeks. The goal is to prevent bacteria from colonizing and reproducing while the damaged site is healing and host defenses regain adequate function to prevent recurrence of infection. For chronic UTIs or recurrent UTIs due to relapses, treatment should be continued for at least three to six weeks. Deep-seated and severe infections, and infections of the kidney or prostate gland, may require more prolonged therapy (Table 4).
Caveat: When treating patients with antimicrobics for long periods, appropriate consideration should be given to adverse side effects. For example, sulfadiazine-trimethoprim combinations have been associated with anorexia, lethargy, keratoconjuctivitis sicca, anorexia, anemia and immune-complex reactions. We have identified sulfadiazine in uroliths. Practitioners should be familiar with common adverse events associated with antimicrobics to be able to place their significance in proper perspective.
Monitoring the outcome
How should response to therapy be monitored?
The following recommendations are guidelines only and should not be interpreted as rigid facts.
- 1. With protocols designed to enhance client and patient compliance, select the least expensive, least toxic and most effective antimicrobial agent and begin therapy.
- 2. Selection of the proper antimicrobial drug, dosage, and frequency of administration usually eliminates bacteria from the urine (but not necessarily the adjacent tissue) within two to five days. Therefore, three to five days following initiation of therapy, collect a urine sample by cystocentesis and culture it for bacteria (so-called therapeutic culture or test for efficacy). Evaluation of urine cultures at this time facilitates early recognition of ineffective therapy. Therapy is considered to be successful only if urine does not contain any viable bacteria. Even though there may be viable bacteria in surrounding tissues, the urine should be sterile. Treatment is ineffective and relapse will likely occur if the bacterial colony count has only been reduced (for example from 105 to 102). In this situation, re-evaluate the therapeutic protocol including selection of antimicrobic drugs and their routes and rates of administration.
- 3. Consider evaluation of a urine culture and urinalysis three to five days (or sooner if necessary) prior to the scheduled discontinuation of therapy, especially if prophylactic antibiotics are to be subsequently used to prevent frequent re-infection. Therapy may be discontinued if the urine is sterile and the urine sediment is normal. If results indicate persistent infection, re-evaluation of therapy is essential. In this situation, initiation of prophylactic low-dose antimicrobial therapy is contraindicated.
- 4. Potential causes of poor response are summarized in Table 5.

Table 5
Caveat: Even when the urine contains no viable microbes, hematuria, pyuria and proteinuria associated with compensatory inflammation are likely to be present. Therefore, one cannot rely on examination of urine sediment alone to detect elimination of bacteria following three to five days of therapy since persistence of reduced numbers of bacteria may fall below the limit of detection by light microscopy.
Dr. Osborne, a diplomate of the American College of Veterinary Internal Medicine, is professor of medicine in the Department of Small Animal Clinical Sciences, College of Veterinary Medicine, University of Minnesota.