Cytology for technicians (Proceedings)
In the last ten years, exfoliative cytology has been gaining popularity as a diagnostic aid in veterinary practice.
In the last ten years, exfoliative cytology has been gaining popularity as a diagnostic aid in veterinary practice. Effective use of diagnostic cytology depends upon proper specimen preparation; however, there are far more descriptions of slide interpretation than there are of slide preparation. In this manuscript we therefore emphasize successful techniques for collection of cytologic specimens from a variety of sources. A discussion of smear preparation and staining techniques is also included.
In vivo collection techniques
Cytologic preparations may be made from lesions present in the living animal (in vivo collections) or from masses or lesions which have been excised (in vitro collections). In vivo collections offer several advantages over surgical removal (e.g., significantly lower cost, no general anesthetic risk, etc.) and is recommended whenever feasible. Several different in vivo collection techniques will be described: the majority are variations of fine needle aspiration, aspiration through catheters, or swabbing of superficial lesions.
Fine needle aspiration of superficial masses and lesions, lymph nodes, and internal masses
Fine needle aspiration can be used to collect cytologic specimens from virtually any lesion internal or superficial which can be identified and stabilized during the collection procedure. The only equipment required is a small syringe (5-10cc) and a 1.5-inch fine gauge (20-25) needle. The site to be aspirated is clipped, washed and prepared for minor surgery. Sterility of the needle is maintained, but surgical gloves and surgical drapes are not necessary. General anesthesia or tranquilization is required only in fractious animals.
The needle is attached to the syringe and introduced into the lesion to be aspirated. As negative pressure is applied to the syringe, the needle is redirected through the mass several times. Moving the needle through different areas of the lesion before the negative pressure is removed assures that the entire lesion area is sampled. Negative pressure is then released and after release of the negative pressure the needle is withdrawn from the lesion.
If the sample has been properly collected (assuming the lesion is solid rather than fluid-filled), the majority of the sample will be contained in the hub of the needle. The needle is separated from the syringe, and the syringe is filled with air and re-attached to the needle. The material in the needle is forced onto a series of microscope slides and finished preparations are made by placing a clear slide across the aspirated material, allowing the material to spread between the two slides, and then gently drawing the top slide across the surface of the bottom slide. The finished "squash preparations" are allowed to air dry and are then stained with a standard hematologic stain (Wright's, Wright's-Giemsa, Diff-Quick, etc.).
Thoracocentesis and abdominal paracentesis
Thoracocentesis and abdominal paracentesis are modifications of basic fine needle aspiration technique. For abdominal paracentesis an eighteen-gauge 1.5-inch needle and 10-20cc syringes are used; similar equipment is used for thoracocentesis and a three-way value is added.
Thoracocentesis may be done on either the left or right side, generally in the 68th rib space. Local anesthesia is recommended with this procedure so that the animal does not move when the thoracic cavity is entered. Movement pre-disposes to lung laceration. The three-way value is employed so that all of the fluid in the chest may be removed in order to provide therapeutic relief of dyspnea. At least two tubes of fluid are saved: one sterile tube for microbiologic evaluation and an EDTA tube for cytologic evaluation. The EDTA sample is used for the evaluation of physical parameters (total cell counts, total protein, specific gravity) and cytologic preparations. Both direct smears and sediment smears may be prepared. Direct smears are made by taking a drop of fluid; placing it on a microscope slide and making a push smear as is done with peripheral blood. Sediment smears are made by centrifuging the sample to obtain a sediment, decanting the supernatant, and preparing a push smear from a drop of sediment. The prepared smears are allowed to air dry and are then stained with standard hematologic stains.
Abdominal paracentesis is performed with the animal standing. The needle is introduced just anterior and to the right of the umbilicus. Neither local nor general anesthesia is generally required. A three-way value is not needed for abdominal paracentesis; there is no therapeutic benefit to removing all of the fluid from the abdominal cavity. The fluid collected by abdominal paracentesis is handled in the same manner as fluid obtained by thoracocentesis.
A key to successful fluid collection from the body cavities is careful positioning of the bevel of the needle as the cavity is entered and aspiration begun. The bevel of the needle should always be directed outward towards the body wall. This prevents plugging of the needle opening by either omental fat or lung. If initial aspiration yields a dry tap, negative pressure is released, the syringe is rotated a full 360° and aspiration is again attempted.
In vitro collection techniques
After a mass has been removed, cytology can still be used to make a diagnosis or to provide useful information while waiting for histopathologic interpretation. Removed masses can be aspirated just as in vivo masses, and squash preparations can be made. Two other techniques are especially suited for excised masses: impression smears and scrapings.
Impression smear technique
There are three keys to making good impression smears: 1) always use a freshly cut surface which has been blotted dry on an absorbent surface, 2) make sure that the cut surface is small in area (less than 1.0cm2) and 3) make multiple imprints on the same slide. Imprints are made by gently touching the cut surface of a mass or lesion to a stationary microscope slide and then lifting the tissue away vertically without allowing any smudging. Two to three rows of imprints (8-15 imprints) can be made/slide and several slides can be made from the same cut surface.
Not all cut surfaces will exfoliate cells readily. Tissues composed of epithelial cells or discrete round cells (lymph node, spleen, mast cell tumor, etc.) can be easily imprinted, but masses composed of connective tissues generally are not amendable to impression smear cytology. When imprinting is not successful, scraping techniques can be utilized.
Scraping technique
Scraping preparations are made by scraping a freshly cut surface with a scalpel blade. The material which accumulates on the blade is then gently streaked the full length of a microscope slide. Only a single streak is made; the blade is not moved back and forth through the smeared material. The slide is air-dried and stained in the standard way.
Cytologic staining technique
Once cytologic preparations have been made, they must be appropriately stained before they can be interpreted. Several different stains and procedures have been advocated historically. The easiest cytologic stain to use is new methylene blue, a one step vital stain. New methylene blue is a good nuclear stain which provides ready evaluation of criteria of malignancy. A major disadvantage of the stain is that it is not permanent and therefore cannot be forwarded for a second opinion.
At the other end of the spectrum as far as ease of use is concerned are the papanicolaou type stains. These stains have long been used in human cytology but offer no real advantage in veterinary cytology. These stains require wet-fixation, and several different staining solutions. In addition, they are not particularly effective in staining microorganisms. For these reasons, the author does not advocate their use.
Instead, the author recommends the use of standard hematologic stains: Wright's stain, Wright's-Giemsa, Wright's-Leishmann, etc. In recent years, some rapid and simple modifications of these hematologic stains have been developed (Harleco's Diff-Quik, Camco Quick Stain, etc.) and have revolutionized diagnostic veterinary cytology. Hematologic stains offer several advantages: 1) they are applied to air-dried specimens; 2) they are permanent stains and slides may be forwarded (either stained or simply air-dried); 3) they are good cytoplasmic and nuclear stains and 4) they effectively stain microorganisms.
Several factors should be kept in mind when preparing slides with hematologic stains. First, all slides should be kept away from formalin before they are stained. Even formalin fumes will partially fix slides and interfere with staining. If unstained specimens are to be mailed to a laboratory for processing, they should not even be shipped in the same package with formalin fixed specimens. Also, whenever slides are to be shipped for staining with routine hematologic stains, they should not be fixed in alcohol before mailing. Alcohol fixation should only be done immediately before staining.
Scanning the slide
Once the cytologic specimen has been properly collected and stained, it is ready for examination under the microscope. The principal roles of the cytologist are to recognize and classify inflammatory reactions to identify any etiologic agent which may have caused inflammation, and to recognize the presence of malignant cells.
Cytologic interpretation begins with proper slide evaluation. Different kinds of specimens demand different approaches. For example, fluid smears are made in the same manner as peripheral blood films and consequently have a thick area (near the point of specimen application), a monolayer (towards the center of the smear) and a feathered edge (area most distant from the point of application). When fluid smears are examined, all three areas of the smear are evaluated, first at low magnification and later under roil immersion. Each area is examined for different features. For example, the most important area for evaluation of cell morphology is the monolayer. Large particulate material, large cells, or cells containing phagocytized material are generally found either in the thick portion of the smear or at the feather edge. Consequently, in the case of inflammatory reactions, classification requires mental integration of the relative numbers of the different cell types seen in the monolayer and at the feather edge (see Inflammatory Cytology below). Since neutrophils concentrate in the monolayer and macrophages in the feather edge, it is easy to appreciate that examination in only one area can lead to misinterpretation of cytologic response.
Slides made by squashing, scraping or impression smear techniques also require scanning at low magnification. In these cases, low magnification assessment is done to determine what part of the preparation is best for evaluation of cell morphology under oil. All squashing, scraping and smearing techniques produce slides which vary in sample quality from area to area. Some areas are too thick to allow evaluation of cytologic detail while in other areas, a majority of the cells are broken. A cardinal rule of cytology is that only intact cells can be interpreted, so finding a monolayer area where cells are intact and cytologic detail can be observed is critical.
Whereas in fluid smears one must be concerned about cell distribution between the monolayer and feathered edge, in scrapings and squash preparations from aspirates this is not an issue because cell distribution is generally random. In contrast, cells in high quality impression smears are not randomly distributed but rather reflect cell distribution in the tissue imprinted. For example, aspirates taken from a reactive hyperplastic lymph node will have a random mixture of small lymphocytes, large lymphoblasts and plasma cells (from medullary cords). Consideration of cell distribution in impression smears can therefore provide some information regarding tissue structure.
General approach to cytologic interpretation
As the slide is scanned, cytologic interpretation begins. Because of the wide variety of specimen sources – effusions, lymph nodes, skin masses, internal organs, etc. – the novice at first may be overwhelmed by the variety of cells encountered. However, the beauty of diagnostic cytologic is that regardless of sample source, the general approach to interpretation is the same. This approach is summarized in the flow chart in Figure 1. Interpretation always begins with the answer to one basic question: How many cell types are present on the slide? Fortunately, there are only three possibilities. Either all of the cells are inflammatory (neutrophils, eosinophils, lymphocytes, plasma cells, or monocyte/macrophages), or all are non-inflammatory (of tissue origin), or the cytologic response is complex (both inflammatory and non-inflammatory).
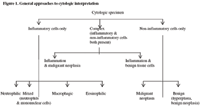
Figure 1. General approaches to cytologic interpretation
Inflammatory cytology
If the reaction is strictly inflammatory then the left side of the flow chart (Figure 1) is followed. The inflammation must first be classified as neutrophilic/eosinophilic, mixed or macrophagic. Classification is done arbitrarily and is based solely upon the relative proportions of the various inflammatory cells in the reaction. If neutrophils (or eosinophils) predominate (greater than 80% of the inflammatory cells present), the reaction is classified as neutrophilic (suppurative or eosinophilic). If neutrophils and mononuclear cells are present in approximately equal numbers (50 to 80% polymorphonuclear, the remaining cells mononuclear) then the response is termed mixed. When mononuclear cells predominate (greater than 50% of the inflammatory cells present) the reaction is classified as macrophagic (nonsuppurative, histiocytic).
Many inflammatory reactions can be even further subdivided on the basis of morphology of the various cell lines. The purpose of subclassification is to provide information regarding pathogenesis of the reaction and to aid in the identification of etiology. A detailed summary of the different classes and subclasses of inflammatory reactions is found in Table 1.
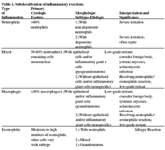
Table 1. Subclassification of inflammatory reactions
Neutrophilic inflammation
Neutrophilic inflammation has two subtypes: neutrophilic with non-degenerate neutrophils, and neutrophilic inflammation with degenerate neutrophils. Non-degenerate neutrophils are either morphologically similar to those of peripheral blood or exhibit nuclear hypersegmentation. Degenerate neutrophils have characteristic nuclear and cytoplasmic changes.
Nuclear changes in degenerate neutrophils include hyalinization and selling of nuclei (an early stage of karyolysis), pyknosis, karyorrhexis, and karyolysis. Pyknotic nuclei are intensely stained and homogeneous without internal chromatin patterns. Karyorrhectic nuclei are generally pyknotic and have been fragmented into small pieces. Karyolysis is the process of spontaneous dissolution of the nucleus and is recognized as the streaming of nuclear chromatin away from the nucleus into the cytoplasm. Karyolysis can only be recognized in intact cells.
Cytoplasmic features of degeneration in neutrophils are generally more subtle than nuclear features. The cytoplasm becomes homogeneous, glassy and somewhat basophilic.
Neutrophilic inflammation with non-degenerate neutrophils is the result of severe irritation. In most cases, etiologic agents are not observed and the aspirated material will be sterile upon cultural examination. Classification of a reaction as neutrophilic with non-degenerate neutrophils is based upon the morphology of the majority of neutrophils; occasional dead neutrophils with pyknotic and karyorrhectic nuclei are expected eve in the purest response.
Neutrophilic inflammation with degenerate neutrophils indicates that there is not only severe irritation but also that there is destruction of neutrophils. Of all the degenerative changes, nuclear hyalinization and swelling and karyolysis are the most significant because they indicate acute cell death. Acute cell death in neutrophils results for the effects of local toxins. The most common toxins are bacterial, and whenever karyolysis is present sepsis should be suspected even if bacteria are not seen. Culture of these samples is always recommended.
Mixed and macrophagic inflammation
Like neutrophilic inflammation, mixed or macrophagic inflammatory reactions may also be subclassified based upon the morphology of their neutrophils. Interpretation of neutrophil degeneration in these instances is the same as for neutrophilic reactions. In general, however, mixed or macrophagic reactions imply less severe irritation than neutrophilic reactions and the neutrophils are usually either non-degenerate or only mildly degenerate. Of greater importance than neutrophil morphology in mixed and macrophagic inflammation is macrophage morphology.
Typical microphages are large, round to oval cells (20-35μ) with eccentric round to oval or irregular nuclei. Nuclei have a lacy or reticulate chromatin pattern. Cytoplasm is abundant, granular, and faintly eosinophilic. Cytoplasmic vacuoles are present and generally contain phagocytized material. Phagocytized material is in most cases indistinct cellular debris or RBC's; however, in some instances, specific etiologic agents may be observed.
If all macrophages are of this typical morphology then mixed or macrophagic inflammatory reactions cannot be further classified. However, many mixed inflammatory responses also contain epithelioid cells, inflammatory giant cells, or both. Epithelioid cells are morphologically similar to typical macrophages except that their cytoplasm contains either no or only small vacuoles, and phagocytized material is absent. Inflammatory giant cells are large, multinucleated macrophages which may contain phagocytized material or have cytoplasm resembling that of epithelioid cells. Nuclei are discrete, typical macrophage nuclei and may be located peripherally at the cell margin or aggregated centrally.
Whenever epithelioid and/or inflammatory cells are present, a mixed or macrophagic reaction can be further classified as pyogranulomatous or granulomatous, depending upon the prevalence of neutrophils. Whereas classification of a response as mixed or macrophagic suggests nothing specific regarding etiology and may be associated with resolving neutrophilic inflammation as well as with more traditional causes of low-grade irritation, classification as pyogranulomatous or granulomatous suggests that a foreign body, mycotic, actinobacterial or mycobacterial etiology should be considered. Often, these etiologic agents can be demonstrated cytologically.
Eosinophilic inflammation
Inflammatory reactions characterized by noticeable numbers of eosinophils are generally the result of allergic phenomena and merit separate consideration. Causes include parasitic disease, mast cell tumors, asthma and systemic atopic responses. Eosinophils are rarely the principal cell seen; rather they represent a prominent accompaniment of a neutrophilic, macrophagic or mixed inflammatory reaction. Eosinophils in these responses may be quite transient; we have seen a number of parasitic diseases where treatment resulted in a disappearance of eosinophils from the reaction in as few as 48-72 hours. Indeed, in these instances, abatement of the eosinophil numbers is a useful index of response to therapy.
Important considerations in the cytologic evaluation of inflammation
Several points regarding cytologic classification of inflammation are worthy of emphasis at this juncture. First, numbers of the various inflammatory components are always estimated, never counted. Uneven distribution of cells in cytologic specimens makes counting differentials unreliable. Second, inflammatory cytology should not be regarded primarily as an indicator of lesion duration but rather is best regarded as an indicator of the level of tissue irritation. Type of inflammation often provides significant help in identifying underlying etiology. Finally, inflammatory cytology also has utility in following the course of an inflammatory reaction or the response to therapy.
Consider the horse with an acute volvulus. Cytologic evaluation of the peritoneal fluid is commonly used to gauge the stage and severity of the reaction in such patients. The primary problem for the animal with volvulus is ischemia of the gut with eventual ischemic necrosis. Early in the disease, reduced blood flow leads to severe irritation of the involved bowel segment. At this stage cytologic evaluation of peritoneal fluid reveals neutrophilic inflammation with non-degenerate neutrophils and the prognosis for surgical intervention is good. If correction does not occur at this stage, the condition will likely worsen. Stasis of intestinal contents leads to bacterial proliferation and turnover with the release of bacterial toxins. These toxins can seep through the intestinal wall in to the peritoneal cavity. At this stage peritoneal fluid cytology reveals neutrophilic inflammation with degenerating neutrophils and prognosis for surgical corrections is only faire.
With continued hypoxia, integrity of the bowel wall becomes increasingly compromised until ischemic necrosis begins. At this stage bacteria from the bowel gain access to the peritoneal cavity. Initially, the majority of these organisms are quickly phagocytized by neutrophils containing bacteria, or septic peritonitis. Prognosis is guarded. Advancing necrosis results in freer access of organisms to the cavity until the capacity of the inflammatory response to contain the bacteria is overwhelmed. This is reflected cytologically by the presence of a variety of types of bacteria both free in the background of the slide as well as within the cytoplasm of severely degenerate neutrophils. Such a finding cytologically dictates a poor to unfavorable prognosis; these animals generally do not recover.